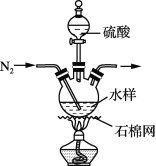
��Ŀ����
����Ŀ��������H2O2�ڷ�Ӧʱ��������Ⱦ�ﱻ��Ϊ����ɫ��������������ܵ�����Խ��Խ��Ĺ�ע��
��.ijʵ��С����H2O2�ֽ�Ϊ����̽��Ũ�ȡ���������Һ����ԶԷ�Ӧ���ʵ�Ӱ�졣�ڳ����°������±���ʾ�ķ������ʵ�顣

��1��ʵ��ٺ͢ڵ�Ŀ����___________��ͬѧ�ǽ���ʵ��ʱû�й۲쵽������������ó����ۡ�������ʾ��ͨ��������H2O2�ȶ������ֽ⡣Ϊ�˴ﵽʵ��Ŀ�ģ����ԭʵ�鷽���ĸĽ�������__________����һ�ַ������ɣ���
��2��ʵ��ۢܢ��У�������������������ʱ��仯�Ĺ�ϵ��ͼ1��ʾ��������ͼ�ܹ��ó���ʵ�������_______________��



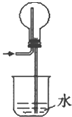
��.������ʾ��ijЩ�������ӻ�����������H2O2�ķֽ�������á�Ϊ�Ƚ�Fe3����Cu2����H2O2�ֽ�Ĵ�Ч������ʵ��С���ͬѧ�������ͼ2��ʾ��ʵ��װ�ý���ʵ�顣
��1��ijͬѧͨ���ⶨO2��������Ƚ�H2O2�ķֽ����ʿ�����ʵ��ʱ����ͨ������_________��________���Ƚϡ�
��2��0.1 g MnO2��ĩ����50 mL H2O2��Һ�У��ڱ�״���·ų�����������ʱ��Ĺ�ϵ��ͼ3��ʾ������ͻ�ѧ��Ӧ���ʱ仯��ԭ��_______�������H2O2�ij�ʼ���ʵ���Ũ��Ϊ__________��������λ��Ч���֣���Ϊ̽��MnO2�ڴ�ʵ���ж�H2O2�ķֽ�������ã��貹������ʵ�飨����д�������������a._______��b._______��
���𰸡�̽��Ũ�ȶԷ�Ӧ���ʵ�Ӱ�� ��Ӧ���м������ͬ�ִ�������ʢ�з�Ӧ����Թܷ���ͬһ��ˮԡ�У� ���Ի���������H2O2�ֽ�����ʣ����Ի����ܼ�СH2O2�ֽ������ ��λʱ������O2����� ���ɵ�λ���O2����Ҫ��ʱ�� ���ŷ�Ӧ�Ľ��У���Ӧ��Ũ�ȼ�С����Ӧ���ʼ��� 0.11 mol��L��1 MnO2��������û�иı� MnO2�Ļ�ѧ������û�иı�
��������
��(1)ʵ��ٺ͢ڵ�Ũ�Ȳ�ͬ��Ϊ�˱��ڱȽϣ�Ӧ����ͬ������������һ���������Ƚϣ�
(2)��ͼ��֪���ݵķ�Ӧ������ܵķ�Ӧ������С���Դ������
��(1)ʵ��ʱ����ͨ��������λʱ������O2����������ɵ�λ���O2����Ҫ��ʱ�����Ƚϣ�
(2)���ŷ�Ӧ�Ľ��У���Һ��Ũ�����ͣ���Ӧ������С������ͼ����Կ���H2O2��ȫ��Ӧ�ų�60 mL O2������2H2O2 ![]() 2H2O+O2�����м��㣻���ݴ����ڷ�Ӧǰ����������ѧ���ʲ�����
2H2O+O2�����м��㣻���ݴ����ڷ�Ӧǰ����������ѧ���ʲ�����
��.(1)ʵ��ٺ͢ڵ�Ũ�Ȳ�ͬ�����ʵ���Ŀ��Ϊ̽��Ũ�ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죻Ϊ�˱��ڱȽϣ�Ӧ����ͬ������������һ���������Ƚϣ�����Ӧ���м������ͬ�ִ�����ʢ�з�Ӧ����Թܷ���ͬһ��ˮԡ�У�
(2)��ͼ��֪���ݵķ�Ӧ������ܵķ�Ӧ������С�����ʵ�鷽����֪�����Ի���������H2O2�ֽ�����ʣ����Ի����ܼ�СH2O2�ֽ�����ʣ�
��.(1)ͨ���ⶨO2��������Ƚ�H2O2�ķֽ����ʿ�����ʵ��ʱ����ͨ��������λʱ������O2����������ɵ�λ���O2����Ҫ��ʱ�����Ƚϣ�
(2)Ũ��Խ��Ӧ����Խ��֮ԽС�����ŷ�Ӧ���У���Ӧ���Ũ����С����������С������ͼ����Կ���H2O2��ȫ��Ӧ�ų�60 mL O2��H2O2�ķֽⷴӦΪ2H2O2![]() 2H2O��O2������n(H2O2)��
2H2O��O2������n(H2O2)��![]() ��2��0.005 36 mol������H2O2�ij�ʼ���ʵ���Ũ��Ϊc(H2O2)��
��2��0.005 36 mol������H2O2�ij�ʼ���ʵ���Ũ��Ϊc(H2O2)��![]() ��0.11 mol��L��1�������Ӧǰ��MnO2������û�иı䣬MnO2�Ļ�ѧ����Ҳû�иı䣬��֤��MnO2�ڴ�ʵ���ж�H2O2�ķֽ�������á�
��0.11 mol��L��1�������Ӧǰ��MnO2������û�иı䣬MnO2�Ļ�ѧ����Ҳû�иı䣬��֤��MnO2�ڴ�ʵ���ж�H2O2�ķֽ�������á�