��Ŀ����
ij������ˮ��Һ�����ܺ������������е������֣�K����Al3����Fe3����Mg2����Ba2����NH4+��Cl����CO32����SO42�����ֱַ�ȡ100 mL�����ȷ���Һ��������ʵ�飺
�ٵ�һ�ݼӹ���NaOH��Һ����ȣ�ֻ�ռ�������0.02 mol���������ɣ�ͬʱ�õ���Һ�ס�
�������Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����պõ�1.02 g���塣
�۵ڶ��ݼ�����BaCl2��Һ�����ɰ�ɫ��������������������ϴ�ӡ�����õ�11.65 g���塣
(1)һ�������ڵ�������________(�����ӷ��ţ���ͬ)��
(2)�ɢٿ�֪��������Ϊ________��Ũ��________���ɢڿ�֪��������Ϊ________��Ũ��________��
�ɢۿ�֪��������Ϊ________��Ũ��________��
(3)K���Ƿ���ڣ�________(��ǡ���)��������___________________________
��(1)Fe3����Mg2����Ba2����CO32����(2)NH4+
0��2 mol/L ��Al3����0.2 mol/L ��SO42����0.5 mol/L
(3)�ǡ����ݵ���غ㣬���������������С�������Ӹ��������������һ����K�����ڡ�
����

 �������ͬ����ϰϵ�д�
�������ͬ����ϰϵ�д���֪���ᡢ��ˮ���ܶ�������ˮ�����Ĺ�ϵ��ͼ��ʾ�����������백ˮ��һ�ݣ�����ݱ�����Ϣ���ش��������⣺
| | ���ʵ����ʵ��� Ũ��/mol��L��1 | ��Һ���ܶ�/g��cm��3 |
| ���� | c1 | ��1 |
| ��ˮ | c2 | ��2 |
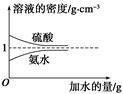
��1�������������������Ϊ________(��д��λ���ú�c1����1�Ĵ���ʽ��ʾ)��
��2�����ʵ���Ũ��Ϊc1 mol��L��1��������ˮ��������(��Ϻ���Һ������仯���Բ���)��������Һ�����ʵ���Ũ��Ϊ________ mol��L��1��
��3�������ʵ���Ũ�ȷֱ�Ϊc2 mol��L��1��
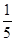 c2 mol��L��1�İ�ˮ��������ϣ�������Һ���ܶ�________(����ڡ�����С�ڡ����ڡ�����ͬ)��2 g��cm��3��������Һ�����ʵ���Ũ��________
c2 mol��L��1�İ�ˮ��������ϣ�������Һ���ܶ�________(����ڡ�����С�ڡ����ڡ�����ͬ)��2 g��cm��3��������Һ�����ʵ���Ũ��________ 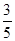 c2 mol��L��1(���Ϻ���Һ������仯���Բ���)��
c2 mol��L��1(���Ϻ���Һ������仯���Բ���)�� ij�������ﺬNH4I��NaHCO3��AlCl3��MgBr2��FeCl2�еļ���,Ϊȷ���ù�������ijɷּ�����ɳɷֵ����ʵ���֮��,�ֽ�������ʵ�顣
ʵ���:
(1)��ɫ����Ϊ ��
(2)�ù�������ijɷ�Ϊ ��
ʵ���:ȡһ�����ĸù�����������ˮ���1 L��Һ,����û����Һ��ͨ��һ������Cl2,�����Һ�м���������(�ֱ���A-��B-��C-��ʾ)�����ʵ�����ͨ��Cl2����Ĺ�ϵ�����ʾ��
| Cl2����� (��״����)/L | 2.8 | 5.6 | 11.2 |
| n(A-)/mol | 1.25 | 1.5 | 2 |
| n(B-)/mol | 1.5 | 1.4 | 0.9 |
| n(C-)/mol | a | 0 | 0 |
(3)a= ��
(4)ԭ���������и���ɳɷֵ����ʵ���֮��Ϊ ��
 2C(g)������2�����C��Ũ��Ϊ0.6mol/L��
2C(g)������2�����C��Ũ��Ϊ0.6mol/L��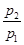 ��0.64����Ӧǰ������ж�������ʵ��������� ��
��0.64����Ӧǰ������ж�������ʵ��������� ��