��Ŀ����
����Ŀ��±�����������ܼ�����Mg��Ӧ���Ƶø����Լ��������Լ����л��ϳɷ�����;�㷺����RΪ����,��֪��![]()
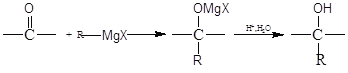
ij�л���A������ת����ϵ��
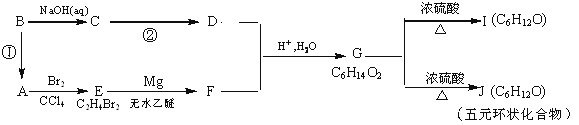
�Իش��������⣺
��1�� ��Ӧ�ٵķ�Ӧ�Լ���ʵ��������____________________
��2�� C��D�Ļ�ѧ��Ӧ����ʽΪ___________________________��
��3�� G�Ľṹ��ʽ��______________________��G�����ͬϵ���������_______
��4��I�����������ŵ�������_________________________
��5���ܷ���������Ӧ��J��ͬ���칹����____�֡�д�����к�-CH3���������칹��Ľṹ��ʽ__________________ .
���𰸡����������Ҵ���Һ������2CH3CH2OH+O2![]() 2CH3CHO+2H2O CH3CH(OH)CH2CH2CH(OH)CH3�Ҷ���̼̼˫�����ǻ�8CH3C(CH3)2CH2CHO[��CH3CH2C��CH3��2CHO����CH3��2CHCH��CH3��CHO]
2CH3CHO+2H2O CH3CH(OH)CH2CH2CH(OH)CH3�Ҷ���̼̼˫�����ǻ�8CH3C(CH3)2CH2CHO[��CH3CH2C��CH3��2CHO����CH3��2CHCH��CH3��CHO]
��������
��Aת��ΪE�ķ�Ӧ������E�ķ���ʽ������֪AΪCH2=CH2��EΪBrCH2CH2Br��FΪBrMgCH2CH2MgBr�����G�ķ���ʽ��Ϸ�Ӧ��Ϣ������֪DΪCH3CHO����CΪ�Ҵ���BΪ±����CH3CH2X����Ϸ�Ӧ��Ϣ��֪GΪ![]() ��JΪ��Ԫ��״�����Ӧ��G�������ǻ��ѳ�һ����ˮ���ò���
��JΪ��Ԫ��״�����Ӧ��G�������ǻ��ѳ�һ����ˮ���ò���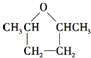 (����Ũ����ʹ�Ҵ���ˮ��������)���Ա�G��I�ķ���ʽ��֪��IΪG��Ũ���������·�����ȥ��Ӧ�IJ����IΪCH3CH(OH)CH2CH=CHCH3��CH3CH(OH)CH2CH2CH=CH2���ݴ˽��н��
(����Ũ����ʹ�Ҵ���ˮ��������)���Ա�G��I�ķ���ʽ��֪��IΪG��Ũ���������·�����ȥ��Ӧ�IJ����IΪCH3CH(OH)CH2CH=CHCH3��CH3CH(OH)CH2CH2CH=CH2���ݴ˽��н��
(1)��Ӧ����CH3CH2X������ȥ��Ӧ����CH2=CH2����Ӧ�Լ���ʵ������Ϊ�����������Ҵ���Һ�����ȣ��ʴ�Ϊ�����������Ҵ���Һ�����ȣ�
(2)C��D�Ļ�ѧ��Ӧ����ʽΪ��2CH3CH2OH+O2 ![]() 2CH3CHO+2H2O���ʴ�Ϊ��2CH3CH2OH+O2
2CH3CHO+2H2O���ʴ�Ϊ��2CH3CH2OH+O2![]() 2CH3CHO+2H2O��
2CH3CHO+2H2O��
(3)������������֪��G�Ľṹ��ʽ��![]() ��G�����ͬϵ���������OHCH2CH2OH���������Ҷ������ʴ�Ϊ��
��G�����ͬϵ���������OHCH2CH2OH���������Ҷ������ʴ�Ϊ��![]() ���Ҷ�����
���Ҷ�����
(4)IΪCH3CH(OH)CH2CH=CHCH3��CH3CH(OH)CH2CH2CH=CH2��I��������������̼̼˫�����ǻ����ʴ�Ϊ��̼̼˫�����ǻ���
(5)����ʽ��I��J��ͬ�����ܷ���������Ӧ��˵���ṹ�к���ȩ������д��C5H11-CHO��-C5H11��8�ֽṹ������C5H11-CHOҲ��8�ֽṹ����ຬ-CH33������ṹ��ʽ�ֱ�Ϊ��CH3C(CH3)2CH2CHO��CH3CH2C(CH3)2CHO��(CH3)2CHCH(CH3)CHO���ʴ�Ϊ��8��CH3C(CH3)2CH2CHO(��CH3CH2C(CH3)2CHO��(CH3)2CHCH(CH3)CHO)��

����Ŀ����������ʵ��������������õ��Ľ�����ȷ����
ѡ�� | ʵ����������� | ʵ����� |
A | �����������������Ҵ���Һ���ȣ���������ͨ�����Ը��������Һ�У���ɫ | ����ϩ���� |
B | ���Թ��е�Ũ�������ͭƬ���Ǻý���������ͨ����Ʒ����Һ������������ | ͭƬδ��ĥ |
C | ��ȥCuSO4��Һ��Fe2+���ȼ�����H2O2���ټ�Cu(OH)2����ҺpH=4 | Ksp[Cu(OH)2]>Ksp[Fe(OH)2] |
D | ����ɫֽ������ʢ�����������ļ���ƿ�У����ϲ���Ƭ������������ | ����Ư�ײ���Cl2����ֱ�����õĽ�� |
A. A B. B C. C D. D