��Ŀ����
����Ŀ�������й�ˮ��Һ��һЩ������ȷ���ǣ� ��
A.ijˮ��Һ�У�c(H+)=![]() mol/L������Һ������
mol/L������Һ������
B.��֪���볣��H2CO3��Ka1=4.2��10-7��Ka2=5.6��10-11��HClO��Ka=2.95��10-8����ͬ�¶���ͬŨ����Һ��pH��Na2CO3<NaClO
C.�����£���Na2S��Һ�м���NaCl��Һ��S2-��ˮ��̶Ȳ���Ӱ��
D.����Cl-��Br-��I-�Ļ����Һ�еμ�AgNO3��Һ���Ȳ�����ɫ������˵��c(I-)���
���𰸡�A
��������
A. Kw=c(H+)��c(OH-)����ijˮ��Һ��c(H+)=![]() mol/L��˵������Һ��c(H+)=c(OH-)������Һ�����ԣ�A��ȷ��
mol/L��˵������Һ��c(H+)=c(OH-)������Һ�����ԣ�A��ȷ��
B. CO32-ˮ��ƽ�ⳣ��![]() ��
��![]() ��ˮ��ƽ�ⳣ��
��ˮ��ƽ�ⳣ��![]() ���������ˮ��ƽ�ⳣ��Խ����ͬŨ��ʱ��ˮ��̶�Խ����Һ�ļ���Խǿ������ˮ��ƽ�ⳣ��֪��ˮ��̶�Na2CO3>NaClO������ͬ�¶���ͬŨ����Һ��pH��Na2CO3>NaClO��B����
���������ˮ��ƽ�ⳣ��Խ����ͬŨ��ʱ��ˮ��̶�Խ����Һ�ļ���Խǿ������ˮ��ƽ�ⳣ��֪��ˮ��̶�Na2CO3>NaClO������ͬ�¶���ͬŨ����Һ��pH��Na2CO3>NaClO��B����
C. �����£���Na2S��Һ�м���NaCl��Һ�����߲���Ӧ�����ᵼ����Һ�������ʹ��Һ��c(Na2S)��С����Һ��pH��С������ϡ��ʹS2-ˮ��̶�����C����
D. ���ɳ����Ⱥ�˳�����ܶȻ���������Һ������Ũ�ȶ��йأ�����Cl-��Br-��I-�Ļ����Һ�еμ�AgNO3��Һ���Ȳ�����ɫ����������˵����Һ��c(I-)���D����
�ʺ���ѡ����A��

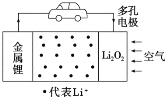
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�