��Ŀ����
����Ŀ����ԴΣ���ǵ�ǰȫ���Ե����⣬����Դ��������Ӧ����ԴΣ������Ҫ�ٴ롣
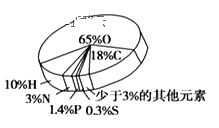
��1������������������Դ����Դ����������___(�����)��
a��������չũ���������������Ľո�ת��Ϊ����Ч����Դ
b����������ú��ʯ�ͺ���Ȼ������������������������Դ����
c������̫���ܡ�ˮ�ܡ����ܡ������ܵ�����Դ������ʹ��ú��ʯ�͵Ȼ�ʯȼ��
d��������Դ���ģ�������Դ���ظ�ʹ�á���Դ��ѭ������
��2�����ʯ��ʯī��Ϊ̼��ͬ�������壬��������������ʱȼ������һ����̼����������ʱȼ�����ɶ�����̼����Ӧ�зų���������ͼ��ʾ��
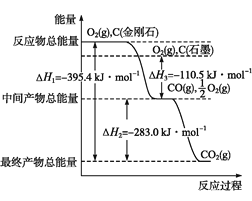
����ͨ��״���£����ʯ��ʯī��___(�������ʯ������ʯī��)���ȶ���ʯī��ȼ����Ϊ___��
��12 gʯī��һ����������ȼ�գ���������36 g���ù��̷ų�������Ϊ___��
��3����֪��N2��O2�����л�ѧ���ļ��ֱܷ���946 kJ��mol1��497 kJ��mol1��
N2(g)+O2(g)![]() 2NO(g)����H=180.0 kJ��mol1��
2NO(g)����H=180.0 kJ��mol1��
NO�����л�ѧ���ļ���Ϊ___ kJ��mol1��
��4���ۺ������й���Ϣ����д��CO��NO��Ӧ���Ȼ�ѧ����ʽ��_________��
���𰸡�acd ʯī 393.5kJ��mol1 252.0kJ 631.5 2NO(g)+2CO(g)�� N2(g)+2CO2(g) ��H=746.0kJ��mol1
��������
(2)��ʯī���������ͣ����ȶ���ʯī��ȼ����ָ1 molʯī��ȫȼ������CO2ʱ�ų���������
��12 gʯī��24 g������Ӧ����1 mol C��0.75 mol O2��Ӧ��������0.5 mol CO��0.5 mol CO2���ų�����0.5 mol��110.5 kJ��mol��1��0.5 mol��393.5 kJ��mol��1��252.0 kJ��(3)��H��E(N2����)��E(O2����)��2E(NO����)��2E(NO����)��946 kJ��mol��1��497 kJ��mol��1��180.0 kJ��mol��1��E(NO����)��631.5 kJ��mol��1��(4)��֪��2CO(g)��O2(g)=2CO2(g) ��H����566.0 kJ��mol��1��N2(g)��O2(g)=2NO(g) ��H��180.0 kJ��mol��1��Ŀ�귴Ӧ2NO(g)��2CO (g)=N2(g)��2CO2(g)����ǰ������ã�����H����746.0 kJ��mol��1��

 ��У����ϵ�д�
��У����ϵ�д�