ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩœ¬±μΈΣ‘ΣΥΊ÷ήΤΎ±μ÷–ΒΎΥΡ÷ήΤΎΒΡ≤ΩΖ÷‘ΣΥΊ![]() ¥”ΉσΒΫ”“Α¥‘≠Ή”–ρ ΐΒί‘ω≈≈Ν–
¥”ΉσΒΫ”“Α¥‘≠Ή”–ρ ΐΒί‘ω≈≈Ν–![]() Θ§ΗυΨί“Σ«σΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ§ΗυΨί“Σ«σΜΊ¥πœ¬Ν–Έ ΧβΘΚ
K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge |
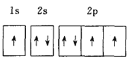
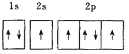
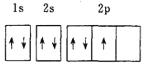
Θ®1Θ©“‘…œ‘ΣΥΊΒΡΜυΧ§‘≠Ή”ΒΡΒγΉ”≈≈≤Φ÷–Θ§4sΙλΒά…œ÷Μ”–1ΗωΒγΉ”ΒΡ‘ΣΥΊ”–______![]() Χν‘ΣΥΊΟϊ≥Τ
Χν‘ΣΥΊΟϊ≥Τ![]() ΓΘ
ΓΘ
Θ®2Θ©“‘…œ‘ΣΥΊ÷–Θ§ τ”Ύs«χΒΡ‘ΣΥΊ”–_________÷÷Θ§ τ”Ύd«χΒΡ‘ΣΥΊ”–______÷÷ΓΘ
Θ®3Θ©ΒΎ“ΜΒγάκΡή![]() ________
________![]() ΧνΓΑ
ΧνΓΑ![]() Γ±ΓΔΓΑ
Γ±ΓΔΓΑ![]() Γ±ΜρΓΑ
Γ±ΜρΓΑ![]() Γ±Θ§œ¬Ά§
Γ±Θ§œ¬Ά§![]() ΓΘ
ΓΘ
Θ®4Θ©œ÷”–Κ§ν―ΒΡΝΫ÷÷―’…ΪΒΡΨßΧεΘ§![]() ΒΡ≈δΈΜ ΐΨυΈΣ6Θ§“Μ÷÷ΈΣΉœ…ΪΘ§“Μ÷÷ΈΣ¬Χ…ΪΘ§œύΙΊ Β―ι÷ΛΟςΘ§ΝΫ÷÷ΨßΧεΒΡΉι≥…Ϋ‘ΈΣ
ΒΡ≈δΈΜ ΐΨυΈΣ6Θ§“Μ÷÷ΈΣΉœ…ΪΘ§“Μ÷÷ΈΣ¬Χ…ΪΘ§œύΙΊ Β―ι÷ΛΟςΘ§ΝΫ÷÷ΨßΧεΒΡΉι≥…Ϋ‘ΈΣ![]() ΓΘΈΣ≤βΕ®’βΝΫ÷÷ΨßΧεΒΡΜ·―ß ΫΘ§…ηΦΤΝΥ»γœ¬ Β―ιΘΚ
ΓΘΈΣ≤βΕ®’βΝΫ÷÷ΨßΧεΒΡΜ·―ß ΫΘ§…ηΦΤΝΥ»γœ¬ Β―ιΘΚ
![]() Ζ÷±π»ΓΒ»÷ ΝΩΒΡΝΫ÷÷≈δΚœΈοΨßΧεΒΡ―υΤΖ≈δ≥…¥ΐ≤β»ή“ΚΘΜ
Ζ÷±π»ΓΒ»÷ ΝΩΒΡΝΫ÷÷≈δΚœΈοΨßΧεΒΡ―υΤΖ≈δ≥…¥ΐ≤β»ή“ΚΘΜ
![]() Ζ÷±πΆυ¥ΐ≤β»ή“Κ÷–ΒΈ»κ
Ζ÷±πΆυ¥ΐ≤β»ή“Κ÷–ΒΈ»κ![]() »ή“ΚΘ§Ψυ≤ζ…ζΑΉ…Ϊ≥ΝΒμΘΜ
»ή“ΚΘ§Ψυ≤ζ…ζΑΉ…Ϊ≥ΝΒμΘΜ
![]() »ή“ΚΖ¥”ΠΒΟΒΫΒΡΑΉ…Ϊ≥ΝΒμ÷ ΝΩΈΣΉœ…ΪΨßΧεΒΡΥ°»ή“ΚΖ¥”ΠΒΟΒΫ≥ΝΒμ÷ ΝΩΒΡ
»ή“ΚΖ¥”ΠΒΟΒΫΒΡΑΉ…Ϊ≥ΝΒμ÷ ΝΩΈΣΉœ…ΪΨßΧεΒΡΥ°»ή“ΚΖ¥”ΠΒΟΒΫ≥ΝΒμ÷ ΝΩΒΡ![]() ΓΘ ‘ΆΤΕœΉœ…ΪΨßΧεΒΡΜ·―ß ΫΈΣ_________ΓΘ
ΓΘ ‘ΆΤΕœΉœ…ΪΨßΧεΒΡΜ·―ß ΫΈΣ_________ΓΘ
Θ®5Θ©Κ§”–‘ΣΥΊKΒΡ―ΈΒΡ―φ…ΪΖ¥”ΠΈΣ__________…ΪΓΘ–μΕύΫπ τ―ΈΕΦΩ…“‘ΖΔ…ζ―φ…ΪΖ¥”ΠΘ§Τδ‘≠“ρ «____________ΓΘ
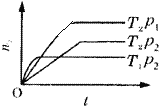
Θ®6Θ©ΝΔΖΫΒΣΜ·≈πΨßΧε![]() Θ§ «“Μ÷÷≥§”≤≤ΡΝœΘ§”–”≈“λΒΡΡΆΡΞ–‘Θ§ΤδΨßΑϊ»γΆΦΥυ ΨΓΘ
Θ§ «“Μ÷÷≥§”≤≤ΡΝœΘ§”–”≈“λΒΡΡΆΡΞ–‘Θ§ΤδΨßΑϊ»γΆΦΥυ ΨΓΘ
»τΝΔΖΫΒΣΜ·≈πΨßΑϊΒΡ±Ώ≥ΛΈΣ![]() Θ§‘ρΝΔΖΫΒΣΜ·≈πΒΡΟήΕ»ΈΣ________g/cm3
Θ§‘ρΝΔΖΫΒΣΜ·≈πΒΡΟήΕ»ΈΣ________g/cm3![]() ÷Μ“Σ«σΝ–Υψ ΫΘ§≤Μ±ΊΦΤΥψ≥ω ΐ÷ΒΘ§ΑΔΖϋΦ”Β¬¬ό≥Θ ΐΒΡ÷ΒΈΣNA)ΓΘ
÷Μ“Σ«σΝ–Υψ ΫΘ§≤Μ±ΊΦΤΥψ≥ω ΐ÷ΒΘ§ΑΔΖϋΦ”Β¬¬ό≥Θ ΐΒΡ÷ΒΈΣNA)ΓΘ
ΓΨ¥πΑΗΓΩΦΊΓΔΗθΓΔΆ≠ 2 8 ![]()
![]()
![]() «≥
«≥![]() Ήœ ΦΛΖΔΧ§ΒΡΒγΉ”¥”ΡήΝΩΫœΗΏΒΡΙλΒά‘Ψ«®ΒΫΡήΝΩΫœΒΆΒΡΙλΒά ±Θ§“‘“ΜΕ®≤®≥Λ
Ήœ ΦΛΖΔΧ§ΒΡΒγΉ”¥”ΡήΝΩΫœΗΏΒΡΙλΒά‘Ψ«®ΒΫΡήΝΩΫœΒΆΒΡΙλΒά ±Θ§“‘“ΜΕ®≤®≥Λ![]() Ω…ΦϊΙβ«χ”ρ
Ω…ΦϊΙβ«χ”ρ![]() ΙβΒΡ–Έ Ϋ ΆΖ≈ΡήΝΩ
ΙβΒΡ–Έ Ϋ ΆΖ≈ΡήΝΩ 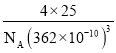
ΓΨΫβΈωΓΩ
![]() ΗυΨίΗς‘≠Ή”ΦέΒγΉ”≈≈≤ΦΖ÷ΈωΘΜ
ΗυΨίΗς‘≠Ή”ΦέΒγΉ”≈≈≤ΦΖ÷ΈωΘΜ
Θ®2Θ©![]() «χΒΡ‘ΣΥΊΈΣΒΎIAΓΔIIAΉε‘ΣΥΊΘΜ d«χΒΡ‘ΣΥΊΈΣ≥ΐΝΥΒΎIBΓΔIIBΉε‘ΣΥΊΆβΒΡΙΐΕ…‘ΣΥΊΘΜ
«χΒΡ‘ΣΥΊΈΣΒΎIAΓΔIIAΉε‘ΣΥΊΘΜ d«χΒΡ‘ΣΥΊΈΣ≥ΐΝΥΒΎIBΓΔIIBΉε‘ΣΥΊΆβΒΡΙΐΕ…‘ΣΥΊΘΜ
![]() ΓΘ
ΓΘ
(4)ΈΜ”ΎΆβΫγΒΡ¬»άκΉ”Ω…”κœθΥα“χ»ή“ΚΖ¥”Π…ζ≥…¬»Μ·“χ≥ΝΒμΘΜ
Θ®5Θ©ΆΗΙΐάΕ…Ϊνή≤ΘΝßΙέ≤λΘ§Κ§”–‘ΣΥΊKΒΡ―ΈΒΡ―φ…ΪΖ¥”ΠΈΣΉœ…ΪΘΜ
Θ®6Θ©![]() ΨßΑϊΒΡΡΠΕϊ÷ ΝΩΓ¬(ΨßΑϊΒΡΧεΜΐΓΝ
ΨßΑϊΒΡΡΠΕϊ÷ ΝΩΓ¬(ΨßΑϊΒΡΧεΜΐΓΝ![]() )ΓΘ
)ΓΘ
![]() ΙλΒά…œ”–“ΜΗωΒγΉ”ΒΡ‘ΣΥΊΈΣΦΊ
ΙλΒά…œ”–“ΜΗωΒγΉ”ΒΡ‘ΣΥΊΈΣΦΊ![]() Θ§Ηθ
Θ§Ηθ![]() Θ§Ά≠
Θ§Ά≠![]() ΘΜ
ΘΜ
![]() «χΒΡ‘ΣΥΊΈΣΒΎIAΓΔIIAΉε‘ΣΥΊΘ§Υυ“‘ΗΟ÷ήΤΎ±μ÷– τ”Ύs«χΒΡ‘ΣΥΊ”–ΘΚKΓΔCaΘ§Ι≤2÷÷ΘΜ d«χΒΡ‘ΣΥΊΈΣ≥ΐΝΥΒΎIBΓΔIIBΉε‘ΣΥΊΆβΒΡΙΐΕ…‘ΣΥΊΘ§ΗΟ÷ήΤΎ±μ÷–”–ScΓΔ TiΓΔ VΓΔ CrΓΔMnΓΔFeΓΔ CoΓΔNiΙ≤8÷÷‘ΣΥΊΘΜ
«χΒΡ‘ΣΥΊΈΣΒΎIAΓΔIIAΉε‘ΣΥΊΘ§Υυ“‘ΗΟ÷ήΤΎ±μ÷– τ”Ύs«χΒΡ‘ΣΥΊ”–ΘΚKΓΔCaΘ§Ι≤2÷÷ΘΜ d«χΒΡ‘ΣΥΊΈΣ≥ΐΝΥΒΎIBΓΔIIBΉε‘ΣΥΊΆβΒΡΙΐΕ…‘ΣΥΊΘ§ΗΟ÷ήΤΎ±μ÷–”–ScΓΔ TiΓΔ VΓΔ CrΓΔMnΓΔFeΓΔ CoΓΔNiΙ≤8÷÷‘ΣΥΊΘΜ
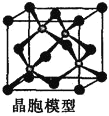
![]() ß»Ξ“ΜΗωΒγΉ”ΚσΘ§Τδ4dΙλΒά¥οΒΫΫœΈ»Ε®ΒΡΑκ≥δ¬ζΉ¥Χ§Θ§Ι Τδ±»
ß»Ξ“ΜΗωΒγΉ”ΚσΘ§Τδ4dΙλΒά¥οΒΫΫœΈ»Ε®ΒΡΑκ≥δ¬ζΉ¥Χ§Θ§Ι Τδ±»![]() “Ή ß»Ξ“ΜΗωΒγΉ”Θ§‘ρΒΎ“ΜΒγάκΡήΘΚ
“Ή ß»Ξ“ΜΗωΒγΉ”Θ§‘ρΒΎ“ΜΒγάκΡήΘΚ![]()
![]() ΘΜ
ΘΜ
![]() ΒΡ≈δΈΜ ΐΨυΈΣ6Θ§Άυ¥ΐ≤β»ή“Κ÷–ΒΈ»κ
ΒΡ≈δΈΜ ΐΨυΈΣ6Θ§Άυ¥ΐ≤β»ή“Κ÷–ΒΈ»κ![]() »ή“ΚΘ§Ψυ≤ζ…ζΑΉ…Ϊ≥ΝΒμΘ§‘ρΨυ”–¬»άκΉ”‘Ύ≈δΚœΈοΒΡΆβΫγΘ§ΝΫΖί≥ΝΒμΘ§Ψ≠œ¥Β”Η…‘οΚσ≥ΤΝΩΘ§ΖΔœ÷‘≠¬Χ…ΪΨßΧεΒΡΥ°»ή“Κ”κ
»ή“ΚΘ§Ψυ≤ζ…ζΑΉ…Ϊ≥ΝΒμΘ§‘ρΨυ”–¬»άκΉ”‘Ύ≈δΚœΈοΒΡΆβΫγΘ§ΝΫΖί≥ΝΒμΘ§Ψ≠œ¥Β”Η…‘οΚσ≥ΤΝΩΘ§ΖΔœ÷‘≠¬Χ…ΪΨßΧεΒΡΥ°»ή“Κ”κ![]() »ή“ΚΖ¥”ΠΒΟΒΫΒΡΑΉ…Ϊ≥ΝΒμ÷ ΝΩΈΣΉœ…ΪΨßΧεΒΡΥ°»ή“ΚΖ¥”ΠΒΟΒΫ≥ΝΒμ÷ ΝΩΒΡ
»ή“ΚΖ¥”ΠΒΟΒΫΒΡΑΉ…Ϊ≥ΝΒμ÷ ΝΩΈΣΉœ…ΪΨßΧεΒΡΥ°»ή“ΚΖ¥”ΠΒΟΒΫ≥ΝΒμ÷ ΝΩΒΡ![]() Θ§Ω…÷ΣΉœ…ΪΨßΧεΒΡΜ·―ß Ϋ÷–Κ§3Ηω¬»άκΉ”Θ§Εχ¬Χ…ΪΨßΧεΒΡΜ·―ß Ϋ÷–Κ§2Ηω¬»άκΉ”Θ§Φ¥Ήœ…ΪΨßΧεΒΡΜ·―ß ΫΈΣ
Θ§Ω…÷ΣΉœ…ΪΨßΧεΒΡΜ·―ß Ϋ÷–Κ§3Ηω¬»άκΉ”Θ§Εχ¬Χ…ΪΨßΧεΒΡΜ·―ß Ϋ÷–Κ§2Ηω¬»άκΉ”Θ§Φ¥Ήœ…ΪΨßΧεΒΡΜ·―ß ΫΈΣ![]() ΘΜ
ΘΜ
![]() Κ§”–‘ΣΥΊKΒΡ―ΈΒΡ―φ…ΪΖ¥”ΠΈΣΉœ…ΪΘΜ–μΕύΫπ τ―ΈΕΦΩ…“‘ΖΔ…ζ―φ…ΪΖ¥”ΠΘ§Τδ‘≠“ρ «ΦΛΖΔΧ§ΒΡΒγΉ”¥”ΡήΝΩΫœΗΏΒΡΙλΒά‘Ψ«®ΒΫΡήΝΩΫœΒΆΒΡΙλΒά ±Θ§“‘“ΜΕ®≤®≥Λ
Κ§”–‘ΣΥΊKΒΡ―ΈΒΡ―φ…ΪΖ¥”ΠΈΣΉœ…ΪΘΜ–μΕύΫπ τ―ΈΕΦΩ…“‘ΖΔ…ζ―φ…ΪΖ¥”ΠΘ§Τδ‘≠“ρ «ΦΛΖΔΧ§ΒΡΒγΉ”¥”ΡήΝΩΫœΗΏΒΡΙλΒά‘Ψ«®ΒΫΡήΝΩΫœΒΆΒΡΙλΒά ±Θ§“‘“ΜΕ®≤®≥Λ![]() Ω…ΦϊΙβ«χ”ρ
Ω…ΦϊΙβ«χ”ρ![]() ΙβΒΡ–Έ Ϋ ΆΖ≈ΡήΝΩΘΜ
ΙβΒΡ–Έ Ϋ ΆΖ≈ΡήΝΩΘΜ
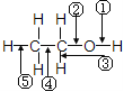
![]() ΨßΑϊ÷–N‘≠Ή” ΐΈΣ4Θ§B‘≠Ή” ΐ
ΨßΑϊ÷–N‘≠Ή” ΐΈΣ4Θ§B‘≠Ή” ΐ![]() Θ§BNΨßΑϊΒΡΡΠΕϊ÷ ΝΩΈΣ
Θ§BNΨßΑϊΒΡΡΠΕϊ÷ ΝΩΈΣ![]() /molΘ§ΝΔΖΫΒΣΜ·≈πΨßΑϊΒΡΧεΜΐ
/molΘ§ΝΔΖΫΒΣΜ·≈πΨßΑϊΒΡΧεΜΐ![]() Θ§“ρ¥ΥΝΔΖΫΒΣΜ·≈πΒΡΟήΕ»
Θ§“ρ¥ΥΝΔΖΫΒΣΜ·≈πΒΡΟήΕ»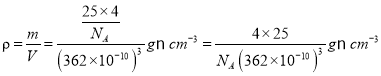 ΓΘ
ΓΘ

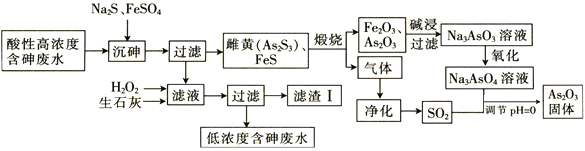
ΓΨΧβΡΩΓΩ’ύ‘ΎΉ‘»ΜΫγ÷–Ζ«≥ΘΖ÷…ΔΘ§ΦΗΚθΟΜ”–±»ΫœΦ·÷–ΒΡ’ύΩσΘ§“ρ¥Υ±Μ»ΥΟ«≥ΤΈΣΓΑœΓ…ΔΫπ τΓ±ΓΘΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©ΜυΧ§’ύ‘≠Ή”ΉνΆβ≤ψΒγΉ”≈≈≤ΦΆΦΈΣ_______Θ§Ge‘≠Ή”ΒΡΒγΉ”ΖΔ…ζ‘Ψ«® ±ΜαΈϋ ’ΜρΖ≈≥ω≤ΜΆ§ΒΡΙβΘ§Ω…”Ο_______![]() Χν“«ΤςΟϊ≥Τ
Χν“«ΤςΟϊ≥Τ![]() …ψ»ΓΤδ‘≠Ή”ΙβΤΉΘ§¥”ΕχΦχΕ®Ge‘ΣΥΊΒΡ¥φ‘ΎΓΘ
…ψ»ΓΤδ‘≠Ή”ΙβΤΉΘ§¥”ΕχΦχΕ®Ge‘ΣΥΊΒΡ¥φ‘ΎΓΘ
Θ®2Θ©’ύ‘ΣΥΊΡή–Έ≥…ΈόΜζΜ·ΚœΈο![]() »γ’ύΥαΡΤΘΚ
»γ’ύΥαΡΤΘΚ![]() ΘΜΕΰ’ύΥαΡΤΘΚ
ΘΜΕΰ’ύΥαΡΤΘΚ![]() ΘΜΥΡ’ύΥαΡΤΘΚ
ΘΜΥΡ’ύΥαΡΤΘΚ![]() Β»
Β»![]() Θ§“≤Ρή–Έ≥…άύΥΤ”ΎΆιΧΰΒΡ’ύΆι
Θ§“≤Ρή–Έ≥…άύΥΤ”ΎΆιΧΰΒΡ’ύΆι![]() ΓΘ
ΓΘ
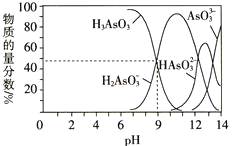
![]() ÷–’ύ‘≠Ή”ΒΡ‘”Μ·ΖΫ ΫΈΣ______________ΓΘ
÷–’ύ‘≠Ή”ΒΡ‘”Μ·ΖΫ ΫΈΣ______________ΓΘ
![]() ’ύ”κΧΦΆ§ΉεΘ§–‘÷ ΦΑΫαΙΙ”–“ΜΕ®ΒΡœύΥΤ–‘Θ§Ψί¥ΥΆΤ≤β
’ύ”κΧΦΆ§ΉεΘ§–‘÷ ΦΑΫαΙΙ”–“ΜΕ®ΒΡœύΥΤ–‘Θ§Ψί¥ΥΆΤ≤β![]() Εΰ’ύΥαΡΤ
Εΰ’ύΥαΡΤ![]() ÷–Κ§”–ΒΡ
÷–Κ§”–ΒΡ![]() ΦϋΒΡ ΐΡΩΈΣ_________ΓΘ
ΦϋΒΡ ΐΡΩΈΣ_________ΓΘ
![]() ÷ΝΫώΟΜ”–ΖΔœ÷n¥σ”Ύ5ΒΡ’ύΆιΘ§ΗυΨίœ¬±μΧαΙ©ΒΡ ΐΨίΖ÷ΈωΤδ÷–ΒΡ‘≠“ρΘΚ___________________ΓΘ
÷ΝΫώΟΜ”–ΖΔœ÷n¥σ”Ύ5ΒΡ’ύΆιΘ§ΗυΨίœ¬±μΧαΙ©ΒΡ ΐΨίΖ÷ΈωΤδ÷–ΒΡ‘≠“ρΘΚ___________________ΓΘ
Μ·―ßΦϋ |
|
|
|
|
ΦϋΡή | 346 | 411 | 188 | 288 |
Θ®3Θ©”–ΜζΕύ‘ΣλΔΥα’ύ≈δΚœΈο «”…![]() Δτ
Δτ![]() ”κ
”κ![]() –Έ≥…ΒΡΘ§ΤδΫαΙΙ»γœ¬ΘΚ
–Έ≥…ΒΡΘ§ΤδΫαΙΙ»γœ¬ΘΚ
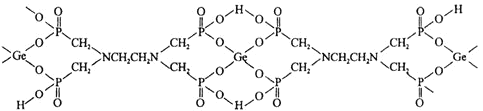
![]() ΗΟ≈δΚœΈο÷–Θ§œ¬Ν–Ής”ΟΝΠ≤Μ¥φ‘ΎΒΡ”–_________
ΗΟ≈δΚœΈο÷–Θ§œ¬Ν–Ής”ΟΝΠ≤Μ¥φ‘ΎΒΡ”–_________
A.ΦΪ–‘Φϋ ![]() Ζ«ΦΪ–‘Φϋ
Ζ«ΦΪ–‘Φϋ ![]() Ϋπ τΦϋ
Ϋπ τΦϋ ![]() ≈δΈΜΦϋ
≈δΈΜΦϋ ![]() «βΦϋ
«βΦϋ ![]() Φϋ
Φϋ
![]() ΗΟ≈δΚœΈο÷–
ΗΟ≈δΚœΈο÷–![]() Δτ
Δτ![]() ΒΡ≈δΈΜ ΐ «_______ΘΜ≈δΈΜ‘≠Ή” «_______
ΒΡ≈δΈΜ ΐ «_______ΘΜ≈δΈΜ‘≠Ή” «_______![]() Χν‘ΣΥΊΖϊΚ≈
Χν‘ΣΥΊΖϊΚ≈![]() ΓΘ
ΓΘ
![]() ‘Ϋβ ΆΝΉΥα
‘Ϋβ ΆΝΉΥα![]() Υα–‘ΈΣ ≤Ο¥”κ―«œθΥαœύΫϋΘΩ______________ΓΘ
Υα–‘ΈΣ ≤Ο¥”κ―«œθΥαœύΫϋΘΩ______________ΓΘ
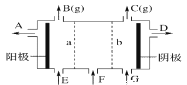
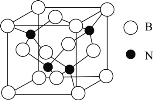
Θ®4Θ©![]() ≥ΘΉςΈΣΨϋ ¬…œΒΡΚλΆβ÷ΤΒΦ≤ΡΝœΘ§ΤδάμœκΨßΑϊ»γΆΦΥυ ΨΓΘ≤βΒΟΨßΑϊ≤Έ ΐ
≥ΘΉςΈΣΨϋ ¬…œΒΡΚλΆβ÷ΤΒΦ≤ΡΝœΘ§ΤδάμœκΨßΑϊ»γΆΦΥυ ΨΓΘ≤βΒΟΨßΑϊ≤Έ ΐ![]() Θ§
Θ§![]() Θ§ΗΟΨßΧεΒΡΟήΕ»ΈΣ_______
Θ§ΗΟΨßΧεΒΡΟήΕ»ΈΣ_______![]() Ν–≥ωΥψ ΫΦ¥Ω…Θ§ΑΔΖϋΦ”Β¬¬ό≥Θ ΐ”Ο
Ν–≥ωΥψ ΫΦ¥Ω…Θ§ΑΔΖϋΦ”Β¬¬ό≥Θ ΐ”Ο![]() ±μ Ψ
±μ Ψ![]() ΓΘ
ΓΘ

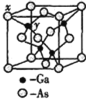
![]() ‘≠Ή”ΒΡΖ÷ ΐΉχ±ξΦ¥ΫΪΨßΑϊ≤Έ ΐaΓΔbΓΔcΨυΩ¥ΉςΓΑ1Γ±ΥυΒΟ≥ωΒΡ»ΐΈ§Ω’ΦδΉχ±ξΘ§“‘1Κ≈ZnΈΣΉχ±ξ‘≠ΒψΘ§‘ρ
‘≠Ή”ΒΡΖ÷ ΐΉχ±ξΦ¥ΫΪΨßΑϊ≤Έ ΐaΓΔbΓΔcΨυΩ¥ΉςΓΑ1Γ±ΥυΒΟ≥ωΒΡ»ΐΈ§Ω’ΦδΉχ±ξΘ§“‘1Κ≈ZnΈΣΉχ±ξ‘≠ΒψΘ§‘ρ![]() ΨßΑϊΆΦ÷–±ξΚ≈ΈΣΓΑ2Γ±ΒΡP‘≠Ή”ΒΡΖ÷ ΐΉχ±ξΈΣ_______ΓΘ
ΨßΑϊΆΦ÷–±ξΚ≈ΈΣΓΑ2Γ±ΒΡP‘≠Ή”ΒΡΖ÷ ΐΉχ±ξΈΣ_______ΓΘ