��Ŀ����
����Ļ�ѧʽ����CaxMgySipO22(OH)2��ʾ��Ҳ����Ca��Mg��Si��H���������ʾ����
��1��ȡ8.l0g�����ĩ���������أ����������0.18g��������Ħ������Ϊ______��
��2����ȡ4.05g�����ĩ����l mol/L������l00mL�г���ܽ⣬���յò���������2. 40g.���ˣ�����Һ��ϴ��Һ�ϲ��������м�����������м���õ�����336mL(��״����).��
��p=_________��
�ڰ���Ļ�ѧʽ�������������ʽ����ʾΪ________��
��1��810g��mol-1����2��8��2CaO��5MgO��8SiO2��H2O
���������������1���ɰ���Ļ�ѧʽCaxMgySipO22(OH)2���Կ���ÿ������������ʱ������Ӧ��ֻ������1��ˮ���ӡ�n(H2O)=m/M=0.18g��18g/mol=0.01mol.,����������ʵ���Ҳ��1mol���������Ħ������Ϊ8.10g��0.01mol=810g/mol.��2��n(����)= 4.05g��810g/mol=0.005mol.���к���Si�����ʵ���Ϊ��2. 40g. ��60g/mol=0.04mol..���ÿĦ���İ����к���Si�����ʵ���Ϊ0.04mol��0.005mol=8.�ɷ���ʽFe+2HCl=FeCl2+H2����֪��n(H2)=0.336L��22.4L/mol=0.015mol.��������Ӧ���������������ʵ���Ϊ0.03mol.�������ĵ�HCl�����ʵ���Ϊ1mol/L��0.1L-0.03mol=0.07mol.��ôÿĦ���İ���Ӧ��Ҫ���ĵ�HCl�����ʵ���Ϊ0.07mol��0.005mol=14������Ca��Mg����+2�۵Ľ��������������ᷴӦʱÿ�����������Ӷ�Ҫ���2��Cl-.����n(Ca2+)+n(Mg2+)=14��2=7.��X+Y=7.�ٽ����Է�������40X+24Y+28��8+16��22+17��2=810.��ʽ������⡣�ɵ�X=2��Y=5�����Ըû�����ķ���ʽΪCa2Mg5Si8O22(OH)2. �����������ʽ����ʾΪ2CaO��5MgO��8SiO2��H2O.
���㣺��������Ħ�������ļ��㡢�����εĻ�ѧʽ��ȷ���������������ʽ�ı�ʾ��֪ʶ��

�輰�仯����������ִ������ķ�չ��������ס���ش������й����⣺
(1)��ԭ�ӵĽṹʾ��ͼ��________��
(2)������Ʒ���豸���õIJ������ڹ����ε���________��
�ٳ�����Ͽˮ���ӡ���ʯӢ���ά�����մ�����
����ͨ�������ݹ�̫���ܵ��
| A���٢ڢ� | B���ۢܢ� | C���ڢۢ� | D���٢ۢ� |
___________________________________________________________��
������ʯʯ�ޡ���Ҫ�ɷ�����������������þ���ᾧˮ�����Ļ�ѧʽ��Mg6[(OH)4Si2O5]2��
��1��������ʯʯ�ޡ�����������ʽΪ______________________������ԭ�Ӱ뾶����Ԫ��
�����ڱ��е�λ����______________________��
��2��Siԭ�ӵ������ĵ����Ų�ʽΪ_____________��SiO2��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ
Ϊ________________________________________________��
��3�� SiCl4��SiO2���۵�_____(��͡������ߡ�)��ԭ����__________________________��
��4������Щ���治���ж�Si��O�ķǽ�����ǿ�� ��
| A������Si��O�����ڱ��е�λ�� |
| B��SiO2��ˮ��������Ӧ |
| C��Si��һ����������������Ӧ������SiO2 |
| D��H2SiO3�����Ա�H2O������ǿ |
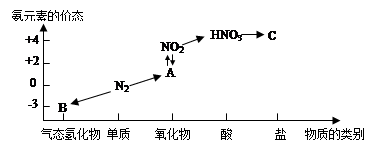
 C
C
 MnCl2 + Cl2��+ 2H2O
MnCl2 + Cl2��+ 2H2O