��Ŀ����
��25mL 0.1mol·L-1NaOH��Һ����μ���0.2mol·L-1CH3COOH��Һ����ҺpH�仯������ͼ��ʾ�������й�����Ũ�ȵıȽ���ȷ����
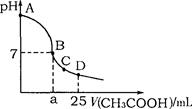
A����A��B����һ��(����A��B��)����Һ�п�����c(Na+)>c(CH3COO��)>c(OH��)>c(H+)
B����B�㣬a>12.5������c(Na+)=c(CH3COO��)=c(OH��)=c(H+)
C����C�㣬c(CH3COO��)>c(Na+)>c(OH��)>c(H+)
D����D�㣬c(CH3COO��)+c(CH3COOH)=c(Na+)

A����A��B����һ��(����A��B��)����Һ�п�����c(Na+)>c(CH3COO��)>c(OH��)>c(H+)
B����B�㣬a>12.5������c(Na+)=c(CH3COO��)=c(OH��)=c(H+)
C����C�㣬c(CH3COO��)>c(Na+)>c(OH��)>c(H+)
D����D�㣬c(CH3COO��)+c(CH3COOH)=c(Na+)
A
�����������AB�����ڣ���Һ�Լ��ԣ�c��OH-����c��H-����������������Һ�ʹ���ǡ�÷�Ӧʱ����ʱ���ɵ���ҺΪ�����ƣ�ˮ���Լ��ԣ�c��OH-����c��CH3COO-����������������Һ�ʹ��ᷴӦ��ʣ������������Һ����Һ��Ȼ�Լ��ԣ���ʱ��ʣ��������������ܴ���c��OH-����c��CH3COO-����Ҳ�п���ʣ����������ƺʹ������д����ˮ��֮��ʣ��Ĵ������Ũ����ȣ�����ѡ��A��ȷ�����a��12.5ʱ��������������Ƶ����ʵ���֮�ȣ�1:1����Ӧ����Һ�����Ϊ�����ƺʹ��ᣬ��Һ���������ԣ���c(OH��)��c(H+)�����ܺ�c(Na+)��c(CH3COO��)��ȣ���Ӧ��С��c(Na+)��c(CH3COO��)����B�����ݵ���غ�c(CH3COO��)��c(OH��)��c(Na+)��c(H+)��֪��ѡ��C�еĹ�ϵ�Ǵ���ģ�ѡ��C����ȷ����D��ʱ����Ӧ�����ʣ�࣬��Һ�����Ϊ��Ũ�ȵĴ���ʹ����ƵĻ������������غ㣬��ʱcCH3COO-����cCH3COOH����2cNa+������D����ȷ����ѡA��
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ����ע�ض�ѧ������֪ʶ���̺�ѵ����ͬʱ�����ض�ѧ�����������������ͷ�����ָ����ѵ����ּ�ڿ���ѧ��������û���֪ʶ���ʵ�����������������������ѧ���������������ʹ���˼ά����������Ĺؼ������úü����غ��ϵ��������غ㡢�����غ��Լ������غ㣬Ȼ����ͼ��������ü��ɡ�

��ϰ��ϵ�д�
 ����ѧ����ϵ�д�
����ѧ����ϵ�д�
�����Ŀ
 ��Cl����H����OH���������ӣ���Һ��һ�����ڣ�
��Cl����H����OH���������ӣ���Һ��һ�����ڣ� )��c(OH��)��c(CO
)��c(OH��)��c(CO )
)
 ��+ c��HC2O
��+ c��HC2O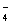 ��
��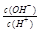 =1��10-8������������ȷ����
=1��10-8������������ȷ����