��Ŀ����
����Ŀ����5 �ֶ�����Ԫ�ص�ԭ��������E��D��B��A��C��˳����������A��Cͬ���ڣ�B��Cͬ���壻A��B���γ����ӻ�����A2B��A2B���������ӵĵ�������ͬ���ҵ�������Ϊ30��D��E���γ�4��10���ӵķ��ӡ��Իش��������⣺
��1��д������Ԫ�ص����ƣ�A_____B_____C_____D______E______��
��2���õ���ʽ��ʾ���ӻ�����A2B���γɹ��̣�_____________________________��
��3��д���������ʵĵ���ʽ��DԪ���γɵĵ���_____________��B��E�γɵĻ�����__________________��A��B��E�γɵĻ�����_______________��D��E�γɵĻ�����_____________��
���𰸡� �� �� �� �� �� 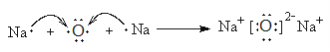
![]()
![]() ��
��![]() 2
2 ![]() �������10��
�������10��![]()
��������5�ֶ�����Ԫ�ص�ԭ��������E��D��B��A��C��˳����������D��E���γ�4��10���ӵķ��ӣ������Ϊ��������EΪH��DΪN��A��B���γ����ӻ�����A2B��A2B���������ӵĵ�������ͬ���ҵ�������Ϊ30����AΪNa��BΪO��A��Cͬ���ڣ�B��Cͬ���壬��֪CΪS��
��1��������������֪��AΪ�ƣ�BΪ����CΪ��DΪ����EΪ�⣻��2��A2BΪNa2O��������Ϊ���ӻ�����õ���ʽ��ʾ���ӻ�����A2B���γɹ���Ϊ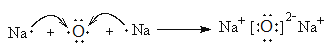 ����3��DԪ���γɵĵ���N2�к��е�������������ʽ���Ա�ʾΪ
����3��DԪ���γɵĵ���N2�к��е�������������ʽ���Ա�ʾΪ![]() ����Ԫ�غ���Ԫ���γɵĻ�����ˮ������Ԫ�غ���Ԫ��֮��ͨ�����ۼ��γɵģ�����ʽΪ��
����Ԫ�غ���Ԫ���γɵĻ�����ˮ������Ԫ�غ���Ԫ��֮��ͨ�����ۼ��γɵģ�����ʽΪ��![]() ���γɵĻ����������˫��ˮ������ʽΪ
���γɵĻ����������˫��ˮ������ʽΪ![]() ��Na��O��H�γɵĻ�������NaOH������ʽΪ
��Na��O��H�γɵĻ�������NaOH������ʽΪ![]() ��H��N�γɵĻ�������NH3������ʽΪ
��H��N�γɵĻ�������NH3������ʽΪ![]() ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�