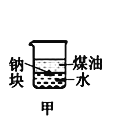
��Ŀ����
����Ŀ��ij��ȤС����������װ���Ʊ�����þ��̽������þ��ijЩ���ʡ�
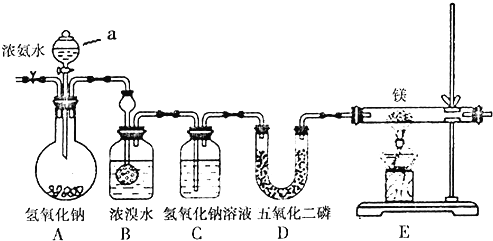
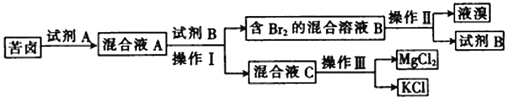
��֪������þ��ˮ������Ӧ��װ��B�з�������Ҫ��ӦΪ��3Br2+2NH3��6HBr+N2��HBr+NH3��NH4Br��
ʵ�鲽�輰����
���������������װ�õ������ԡ�
��ͨ��������塣
�۴�Һ©���������۲쵽B��Ũ��ˮ��ɫ��dz����ɫ��C�в�����ɫ���ݡ�
�ܵ�ȼ�ƾ��ƣ��۲�E�й������˻���ɫ��
�ش��������⣺
��1��Aװ��������a������Ϊ______��ͨ����������Ŀ����__________________��
��2��װ��C��������______________________��
��3��Dװ���е�P2O5��һ�ַ������Ե����Ը�������������岻����Ũ����������P2O5�������______��
a.NH3 b. HI c. SO2 d. CO2
��4��Eװ���з�����Ӧ�Ļ�ѧ����ʽΪ��___________________________��
��5����װ���д���������ȱ�ݣ���ĸĽ���ʩ��_________________________��
���𰸡���Һ©�� �ų�װ���еĿ��� ���յ����л��е�HBr(Br2)���� b N2+3Mg![]() Mg3N2 ��E������װ�м�ʯ�ҵĸ���ܣ���ֹ�����е�ˮ��������Eװ����
Mg3N2 ��E������װ�м�ʯ�ҵĸ���ܣ���ֹ�����е�ˮ��������Eװ����
��������
�������Ʊ�����þ��ʵ������⣬������������������ȡ���������������������õ�����������������Һ���ӷ����壬���������������ﵪ��������ڼ��ȵ������þ�뵪����Ӧ���ɵ���þ���������е�����������
��1��Aװ��������a������Ϊ��Һ©����Ϊ��ֹþ����������Ӧ�������Ҫͨ��������壬�ų�װ���еĿ������ṩ�������������⸱��������ɣ���ͨ����������Ŀ�����ų�װ���еĿ�����
��2�����ӷ������ɵĵ����к����������Լ��廯�⣬��װ��C�����������յ����л��е�HBr(Br2)���壬��ֹ����Ӧ�ķ�����
��3��NH3�����ᷴӦ��HI����ǿ��ԭ�ԣ��ʰ���������Ũ������Ҳ����������������� HI������Ũ�����������������������CO2��SO2�������ö��߸���ʴ�Ϊb��
��4��E�з�����Ӧ�Ļ�ѧ����ʽΪN2+3Mg![]() Mg3N2��
Mg3N2��
��5�����ڵ���þ��ˮ������Ӧ������Ϊ��ֹ����þˮ�⣬��Eװ�ú�Ҫ��һ������װ�ã��������е�ˮ��������Eװ�á�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ������װ����ʾ��ʵ���У����ܴﵽʵ��Ŀ����
|
|
|
|
A����ʱ�俴��Fe(OH)2��ɫ���� | B��֤����(ú��)< ��(��) < ��(ˮ) | C��̽�������ԣ� KMnO4��Cl2��I2 | D���Ƚ�NaHCO3��Na2CO3�����ȶ��� |
A. A B. B C. C D. D