��Ŀ����
ˮ��Һ�д��ڶ���ƽ�⣬�������ѧ��ѧ֪ʶ�ش��������⣺
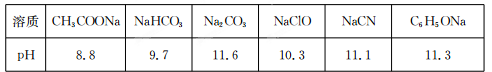
��1����Ũ�Ⱦ�Ϊ0.1mol/L��CH3COONH4 ��NH4HSO4 ��NH3.H2O ��(NH4)2SO4��Һ�У�NH4+Ũ���ɴ�С��˳��Ϊ ������ţ�
��2��Ũ�Ⱦ�Ϊ0.1 mol��L��1��������Һ�������ᡢ�ڴ��ᡢ���������ơ����Ȼ��������Һ����ˮ�������H��Ũ���ɴ�С��˳����(�����) ��
��3�������£���pH=6������ˮ�м���2.3g�����ƣ���ַ�Ӧ���ټ�����ˮϡ�͵�1L��������Һ��pH= ��
��1����>��>��>�ۣ�2����>��>��>�٣�3��11
���������������1����Ũ�Ⱦ�Ϊ0.1mol/L��CH3COONH4 ��NH4HSO4 ��NH3.H2O ��(NH4)2SO4��Һ�У�NH3?H2OΪ������ʣ����������ģ���CH3COONH4��NH4HSO4 ��(NH4)2SO4Ϊǿ����ʣ�ȫ�����룬����NH3.H2O��NH4+Ũ����С��(NH4)2SO4��������NH4+����(NH4)2SO4��NH4+Ũ�����NH4HSO4������������ӣ�NH4+ˮ���ܵ����ƣ�CH3COONH4�д����ˮ��ʼ��ԣ�NH4+ˮ��õ��ٽ�����NH4HSO4��NH4+Ũ�ȴ���CH3COONH4��NH4+Ũ�ȣ���NH4+Ũ���ɴ�С��˳��Ϊ ��>��>��>�ۣ���2�� Ũ�Ⱦ�Ϊ0.1 mol��L��1��������Һ�������ᡢ�ڴ��ᡢ���������ơ����Ȼ��������Һ���Ȼ��Ϊǿ�������Σ�����ˮ�⣬�ٽ�ˮ�ĵ��룬��ˮ�������H��Ũ��������ᡢ��������ˮ�ĵ��룬����Һ�Ƚ�ˮ��������������ӣ��ɱȽ���Һ����������Ũ�ȣ�����Ϊǿ�ᣬȫ�����룬0.1 mol��L��1��������������Ũ��Ϊ0.2mol��L��1��������Ũ��Ϊ5��10��14mol��L��1������Ϊ���ᣬ0.1 mol��L��1������Һ��������Ũ��ԶС��0.1 mol��L��1��������Ũ��Զ����1��10��13mol��L��1��������������ˮ�ĵ��룬����Һ�Ƚ�ˮ��������������ӣ��ɱȽ���Һ�������ӵ�Ũ�ȣ�0.1 mol��L��1����������Һ��������Ũ��Ϊ0.1 mol��L��1����Һ��������Ũ��Ϊ1��10��13mol��L��1������������������Һ����ˮ�������H��Ũ���ɴ�С��˳���Ǣ�>��>��>�٣���3�������£���pH=6������ˮ�м���2.3g�����ƣ�������Ӧ2Na+2H2O=2NaOH+H2��������0.1mol�������ƣ��ټ�����ˮϡ�͵�1L��������Һ��������Ũ��Ϊ0.1 mol��L��1�����¶�������ˮ��pH=6����ˮ�����ӻ�����Ϊ1��10��12������Һ��������Ũ��Ϊ1��10��11mol��L��1��pH=11��
���㣺����������Һ���漰ˮ�ĵ��롢����ˮ�⼰PH���㡣

 ��У����ϵ�д�
��У����ϵ�д�����Ⱦ��Ҫ�����ڸ���Ŀ��ɺ�ұ���������ų��ĸ����ɷ�ΪSiO2�� A12O3��MgO�� Fe2O3��Na2Cr2O7�ȣ�����Σ�����Ļ�ѧ�ɷ���Na2Cr2O7����֪���� Na2Cr2O7������ˮ����ǿ���������� +6�۸��ױ��������գ����°���+3�۸����ױ��������գ�����С��������ʵ����ģ�����������������Ⱦ�Ĺ����������£����ֲ����������ԣ���
��1������������У�ϡH2SO4�ܽ�A12O3�����ӷ���ʽ�� ��
��2������1�����Һ�д�������ƽ�⣺Cr2O72-����ɫ��+ H2O  2CrO42-����ɫ��+ 2H+����ʱ��ҺӦ���ֵ���ɫ�� ��
2CrO42-����ɫ��+ 2H+����ʱ��ҺӦ���ֵ���ɫ�� ��
��3����FeSO4��7H2O��ԭ Na2Cr2O7�����ӷ���ʽ�� ��
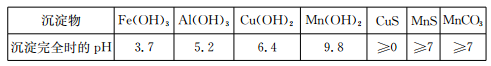
��4����֪�������������������pH
| ���� | Fe(OH)3 | Al(OH)3 | Cr(OH)3 | Fe(OH)2 | Mg(OH)2 |
| ��ʼ���� | 1��5 | 3��4 | 4��9 | 6��3 | 9��6 |
| ��ȫ���� | 2��8 | 4��7 | 5��5 | 8��3 | 12��4 |
���ݱ������ݣ������е�aΪ ��
��5����������2�����ʣ�������Ӧ�����ӷ���ʽ�� ��
��6������3������Һ�ֻ���������1������Һ�У���Ŀ���� ��
��ҵ̼����(����ԼΪ98%)�к���Ca2����Mg2����Fe3����Cl����SO42�������ʣ��ᴿ�����������£�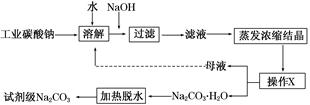
��.̼���Ƶı�����Һ�ڲ�ͬ�¶�����������������ͼ��ʾ��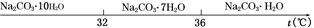
��.�й����ʵ��ܶȻ����£�
| ���� | CaCO3 | MgCO3 | Ca(OH)2 | Mg(OH)2 | Fe(OH)3 |
| Ksp | 4.96��10��9 | 6.82��10��6 | 4.68��10��6 | 5.61��10��12 | 2.64��10��39 |
�ش��������⣺
(1)����NaOH��Һ����˵õ�����������Ҫ����________(��д��ѧʽ)��25��ʱ������Mg2����Fe3������Һ�еμ�NaOH��Һ�������ֳ�����������Һ��pH��8 ʱ��c(Mg2��)��c(Fe3��)��________��
(2)����XΪ________�����¶�Ӧ������_____________________________________
(3)���˴ӡ���ɫ��ѧ���Ƕ����뽫��ĸҺ����������������ʾ����ѭ��ʹ�á��������ʵ�ʹ�ҵ�������Ƿ����________����˵������______________________________
________________________________________________________________________��
��ͼ��ʾ������ƿ�зֱ�װ�뺬��̪��0.01 mol��L-1 CH3COONa��Һ,���ֱ������ʢ��ˮ���ձ���,Ȼ�����ձ����м�����ʯ��,���ձ����м���NH4NO3����,�ձ����в����κ����ʡ�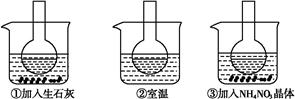
(1)����̪��0.01 mol��L-1 CH3COONa��Һ��dz��ɫ��ԭ��Ϊ
(2)ʵ������з�����ƿ������Һ��ɫ����,��ƿ������Һ��ɫ��dz,������������ȷ��������������
| A��ˮ�ⷴӦΪ���ȷ�Ӧ | B��ˮ�ⷴӦΪ���ȷ�Ӧ |
| C��NH4NO3����ˮʱ�ų����� | D��NH4NO3����ˮʱ�������� |
�������ճ�����������ĵ�ζ������Ҫ�Ļ���ԭ�ϣ����������䳣�����Ρ�
��1����������Һ�и�����Ũ���ɴ�С��˳��Ϊ ��
��2�����ڴ�����Һ�ʹ�������Һ������˵����ȷ���� ������ĸ����ͬ����
| A��ϡ�ʹ�����Һ������ĵ���̶�����ϡ�ʹ�������Һ������Ƶ�ˮ��̶ȼ�С |
| B�������¶ȿ��Դٽ�������룬�������¶Ȼ����ƴ�����ˮ�� |
| C������ʹ����ƵĻ��Һ�У��������ƴ����Ƶ�ˮ�⡢������Ҳ���ƴ���ĵ��� |
| D������ʹ����ƵĻ��Һ�У�����ٽ������Ƶ�ˮ�⡢������Ҳ�ٽ�����ĵ��� |
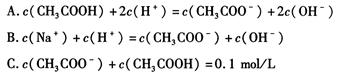

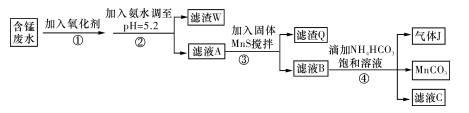
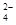 ��H+��Fe2+��Al3+��Cu2+�����Ʊ������ܴ��Բ���̼���̣�MnCO3��������һ�ֹ�ҵ�������£�
��H+��Fe2+��Al3+��Cu2+�����Ʊ������ܴ��Բ���̼���̣�MnCO3��������һ�ֹ�ҵ�������£�