��Ŀ����
4���õ�ʯ�Ʊ�����Ȳ�����г�����������H2S���壮��ͼ�е�������ҩƷ�����һ���Ʊ���������Ȳ��װ�ã�����ͨ���ⶨ��Ȳ�������Ӷ������ʯ���ȣ���ش������й����⣺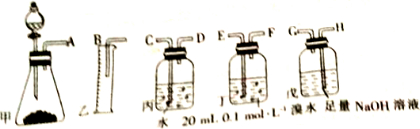
��1������ʵ��ʱ���������尴��������������������ȷ����˳����AHGEFDCB����ӿ���ĸ����
��2��Ϊ��ʹʵ��������ƽ�ȣ����з�Һ©�����Һ��ͨ���ñ���ʳ��ˮ��
��3�����ڱ�״������ˮ����Ȳ��ȫ��Ӧ����C2H2Br4����֪��ȡ��ʯmg�������Ͳ��Һ�����VmL�����ʯ���ȿɱ�ʾΪ$\frac{��0.001+\frac{V}{22400}����64}{m}$��100%��
��4����û�г�H2S��װ�ã��ⶨ�������ƫ�ߣ��ƫ�ߡ���ƫ�͡�����
���� ��1����ʯ������ˮ��Ӧ������Ȳ�����л��е�H2S����NaOH��Һ���գ���Ȳ���屻��ˮ���պ����µIJ���ͨ����ˮ�������������
��2��ʵ����һ���ñ���ʳ��ˮ����ˮ����ʵ�飬�Ի��ƽ�ȵ���Ȳ������
��3�������嵥�ʵ����ʵ���������ĵ���Ȳ�������ٸ���ˮ����������Ȳ�������Ȼ�������Ȳ���̼���Ƶ�������������������
��4��������л�ԭ�ԣ��ܹ����巢��������ԭ��Ӧ�����²����Ȳ�����ʵ���ƫ�࣮
��� �⣺��1����ʯ������ˮ��Ӧ������Ȳ�����л��е�H2S����NaOH��Һ���գ���Ȳ���屻��ˮ���պ����µIJ���ͨ����ˮ�������������������ȷ������˳��Ϊ��AHGEFDCB��
�ʴ�Ϊ��AHGEFDCB��
��2��ʵ����һ���ñ���ʳ��ˮ����ˮ����ʵ�飬�Ի��ƽ�ȵ���Ȳ������
�ʴ�Ϊ������ʳ��ˮ��
��3����ˮ����Ȳǡ����ȫ��Ӧ����C2H2Br4���嵥�ʵ����ʵ���Ϊ0.1mol/L��0.02L=0.002mol�����ĵ���ȲΪ��0.001mol�����������Ͳ��Һ�����ΪVmL�������������Ȳ�����ΪVml�������ʵ���Ϊ$\frac{V��1{0}^{-3}}{22.4L/mol}$=$\frac{V}{22400}$mol��
������Ȳ�������ʵ���Ϊ0.001mol+$\frac{V}{22400}$mol��
��CaC2+2H2O-��Ca��OH��2+C2H2����֪CaC2�����ʵ���0.001mol+$\frac{V}{22400}$mol������Ϊ��0.001+$\frac{V}{22400}$��64g�����Ե�ʯ���ȿɱ�ʾΪ$\frac{��0.001+\frac{V}{22400}����64}{m}$��100%��
�ʴ�Ϊ��$\frac{��0.001+\frac{V}{22400}����64}{m}$��100%��
��4��������л�ԭ�ԣ��ܹ����巢��������ԭ��Ӧ�����²����Ȳ�����ʵ���ƫ�࣬���ղ�õ�ʯ�Ĵ���ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
���� ����Ϊʵ���⣬��������Ȳ���Ʊ�����Ȳ�����ʣ�ʵ�����ݵķ����ͼ��㣬��Ϥʵ����̺�ԭ���ǽ���ؼ�����Ŀ�Ѷ��еȣ�

| A�� | 13C��15N������ͬ�������� | B�� | 13C��C60��ͬһ������ | ||
| C�� | 15N��14N��Ϊͬλ�� | D�� | 15N�ĺ������������������ͬ |
| A�� | ������ | B�� | �屽 | C�� | ���Ȼ�̼ | D�� | �ױ� |
| A�� |  | B�� |  | C�� |  | D�� |  |
| A�� | M��N��ͬ�������壬��M=N����H=+119kJ/mol��֪��N��M�ȶ� | |
| B�� | Na2O2�ĵ���ʽ�� | |
| C�� | ��ʾ��Ȳ��ȼ���ȡ���Ӧ���Ȼ�ѧ����ʽ��C2H2 ��g��+$\frac{5}{2}$O2 ��g���T��2CO2 ��g��+H2O��g����H=-1 256kJ/mol | |
| D�� | �����ǵ�ʵ��ʽ��CH2O |