��Ŀ����
������A�����ֲ�ͬ������Ԫ��X��Y��ɣ��������õ��Ե�ԡ�w.w.w.k.s.5.u.c.o.m
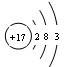
��1��X�ĵ��ʼȿ������ᷴӦ���ֿ���NaOH��Һ��Ӧ��X��ԭ�ӽṹʾ��ͼΪ ��
��2��0.1 mol��L X����������Һ��0.1 mol��L NaOH��Һ�������ϣ���Ӧ�����ӷ���ʽΪ
��3��Y�ĵ����ڷŵ�����������������Ӧ��д����ѧ����ʽ��
��
��4��������A����ˮ������Ӧ���ɺ�Y�Ļ�����Z��Z�����к���10�����ӡ�д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��5����ҵ��X�ĵ��ʺͻ�����Z�ڸ���1700K��Ӧ�Ʊ�A����֪�÷�Ӧ������Ϊ���û���Ӧ���÷�Ӧ�Ļ�ѧ����ʽ�� ��
����10�֣�
(1)
(2) Al3+ + 3OH- �� Al(OH)3��
(3) N2 + O2 ![]() 2NO
2NO
(4) AlN + 3H2O �� Al(OH)3��+ NH3��
(5) 2Al + 2NH3 ![]() 2AlN + 3H2
2AlN + 3H2

��ϰ��ϵ�д�
 �����ܾ�ϵ�д�
�����ܾ�ϵ�д� ���ƿ�����ϵ�д�
���ƿ�����ϵ�д� ���¿쳵����������ϵ�д�
���¿쳵����������ϵ�д�
�����Ŀ


 ��2012?��������ģ���������г�������X��ʯī�Ͷ������ѣ�TiO2����������ϣ������·�Ӧ�õ��Ļ������������Ԫ����ɣ��Ҷ��������մɲ��ϣ��ڻ���͵���������ҪӦ�ã��������������������գ�
��2012?��������ģ���������г�������X��ʯī�Ͷ������ѣ�TiO2����������ϣ������·�Ӧ�õ��Ļ������������Ԫ����ɣ��Ҷ��������մɲ��ϣ��ڻ���͵���������ҪӦ�ã��������������������գ�