��Ŀ����
17��ʵ���ҳ��õ�Ũ�����ܶ�Ϊ1.17g•mL-1����������Ϊ36.5%����1����Ũ��������ʵ���Ũ��Ϊ���٣�
��2��ȡ��Ũ����50mL��������ˮϡ����200mL��ϡ�ͺ���������ʵ���Ũ��Ϊ���٣�
��3����10.6gNa2CO3Ͷ������������ʵ�����õ�ϡ�����У���ַ�Ӧ�ų���CO2�ڱ�״���µ����Ϊ���٣������ɵ�CO2ͨ�뵽��������ʯ��ˮ�У����ɰ�ɫ����������Ϊ����g��
���� ��1������c=$\frac{1000�Ѧ�}{M}$���㣻
��2������ϡ�Ͷ��ɼ��㣻
��3������n=$\frac{m}{M}$����̼�������ʵ������ٸ��ݷ���ʽNa2CO3+2HCl=2NaCl+CO2��+H2O���������ɶ�����̼���ʵ���������V=nVm���������̼���������̼Ԫ���غ����CaCO3�����ʵ������ٸ���m=nM����CaCO3��������
��� �⣺��1������c=$\frac{1000�Ѧ�}{M}$��֪���ܶ�Ϊ1.17g•mL-1����������Ϊ36.5%����������ʵ���Ũ��Ϊ��$\frac{1000��1.17��36.5%}{36.5}$mol/L=11.7mol/L��
�𣺸���������ʵ���Ũ��Ϊ11.7mol/L��
��2������ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����ʵ������䣬��ϡ�ͺ�����Ũ��Ϊ$\frac{0.05L��11.7mol/L}{0.2L}$=2.925mol/L��
��ϡ�ͺ������Ũ��Ϊ2.925mol/L��
��3��10.6gNa2CO3�����ʵ���Ϊ$\frac{10.6g}{106g/mol}$=0.1mol����
Na2CO3+2HCl=2NaCl+CO2��+H2O
0.1mol 0.1mol
�ʱ���£����ɶ�����̼���Ϊ0.1mol��22.4L/mol=2.24L��
����̼Ԫ���غ㣺n��CaCO3��=n��CO2��=0.1mol��������CaCO3������Ϊ0.1mol��100g=10g��
�𣺱�������ɶ�����̼���Ϊ2.24L����������ʯ��ˮ���ղ����Ķ�����̼������CaCO3������Ϊ10g��
���� ���⿼�黯ѧ����ʽ���㡢���ʵ���Ũ�ȼ��㣬�ѶȲ���ע�������������ʵ���Ũ������������֮���ϵ��

 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д� ��ʦָ��һ��ͨϵ�д�
��ʦָ��һ��ͨϵ�д�| A�� | Fe | B�� | Mg | C�� | Na | D�� | Cu |
| A�� | ���� | B�� | �ܶ� | C�� | �������� | D�� | �������� |
| A�� | K+��Mg2+��Cl-��OH- | B�� | K+��Cu2+��SO42-��Na+ | ||
| C�� | NH4+��CO32-��NO3-��Na+ | D�� | NH4+��Cl-��HCO3-��H+ |
| A�� | ��״���£�22.4LH2O���еķ�����ΪNA�� | |
| B�� | ͨ��״���£�NA ��CO2���ӵ����Ϊ22.4L | |
| C�� | ���³�ѹ�£�40gNaOH���е������Ӹ���ΪNA�� | |
| D�� | ���ʵ���Ũ��Ϊ0.5mol/L��BaCL2��Һ�У����������Ӹ���ΪNA�� |
| A�� | ����������Fe3+��H+���ʷ�Ӧ�Ȳ����������������� | |
| B�� | ��Ӧ�����13.44LH2����״���� | |
| C�� | ��Ӧ����Һ��Fe2+��Fe3+���ʵ���֮��Ϊ0.9mol | |
| D�� | ��Ӧ����Һ��Fe3+���ʵ���Ϊ0.8mol |
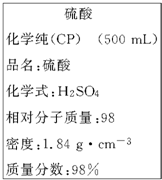 ��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص�Ũ�����Լ���ǩ�ϵIJ������ݣ����ø�Ũ��������200mL 1.0mol/L��ϡ���ᣮ�ɹ�ѡ�õ������У�
��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص�Ũ�����Լ���ǩ�ϵIJ������ݣ����ø�Ũ��������200mL 1.0mol/L��ϡ���ᣮ�ɹ�ѡ�õ������У�