��Ŀ����
����Ŀ��
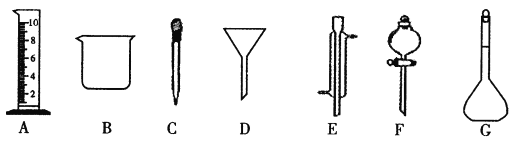
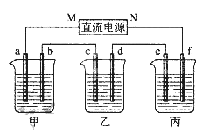
��1��д���������ƣ�E_____________��F_____________��
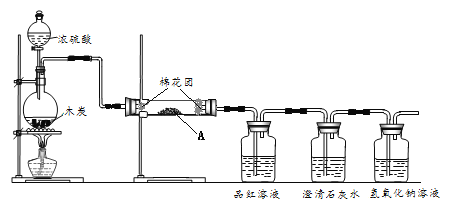
��2������ʵ��������õ�����D����_________(ѡ������ѡ��ı����ĸ )��
A������ˮ��CC14�Ļ���� B������ˮ�;ƾ��Ļ���� C������ˮ����ɰ�Ļ����
��.ij������ȤС����Ҫ200mL1mol/L��Na2CO3��Һ,��ش��������⣺
��1��������Һ����������ҩƷ��
Ӧ��ȡNa2CO3������ | Ӧѡ������ƿ�Ĺ�� | ������ƿ���Ҫ������������������ͼ�е�______��_____�������� |
��2������ʱ������ȷ�IJ���˳����(����ĸ��ʾ��ÿ������ֻ����һ��)______________��
A��������ȴ����Һ�ز�����ע������ƿ��
B����������ƽȷ��������Na2CO3�������������ձ��У��ټ�������ˮ���ò���������������ʹ���ܽ�(��Ҫʱ�ɼ���)
C��������ˮϴ���ձ�2-3�Σ�ϴ��Һ��ע������ƿ�У���
D�����ý�ͷ�ιܼ�ˮ��ʹ��Һ��Һ��ǡ����̶�������
E.������ƿ�ǽ�����ҡ��
F.����������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1��2cm��
��3�������������������������ҺŨ�Ƚ��к�Ӱ��(����ƫ��������ƫ����������Ӱ����)?
û�н���C����________��������Һʱ������ƿδ����_________������ʱ���ӿ̶���_________��
���𰸡�I.��1�������ܣ���Һ©������2��c��
II.��1��26.5��250mL��B�����ձ�����C����ͷ�ιܣ���
��2��B��A��C��F��D��E����3��ƫ�ͣ���Ӱ�죬ƫ�ߡ�
������������I. ��1��E���������������ܣ�F �����Ƿ�Һ©������2������D��©����A������ˮ��CC14�����ֻ������ܵ�Һ������Ҫʹ�÷�Һ©��������©��������B�� ˮ�;ƾ��ǻ��ܵ�Һ��������ߵķе㲻ͬ������ˮ�;ƾ��Ļ����Ҫ������ķ�����C�� ��ɳ������ˮ����˷��������ԵĹ�����Һ������ķ����ǹ��ˣ�ʹ�õ�������©������ѡ��c��ȷ����.����200mL1mol/L��Na2CO3��Һ����������ƿ�Ĺ����250mL����Һ������Ũ����ȣ�����n(Na2CO3)=1mol/L��0.25L=0.25mol��m(Na2CO3)= 0.25mol��106g/mol=26.5g��Ӧѡ������ƿ�Ĺ��250mL��������ƿ���Ҫ������������������ͼ�е��ձ�����ͷ�ιܼ�����������ѡ�������B��C����2�� �������ʵ���Ũ�ȵ���Һʱ�����Ʋ�����B����������ƽȷ��������Na2CO3�������������ձ��У��ټ�������ˮ���ò���������������ʹ���ܽ⣻A��������ȴ����Һ�ز�����ע������ƿ�У�C��������ˮϴ���ձ�2-3�Σ�ϴ��Һ��ע������ƿ�У���F.����������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1��2cm����D�����ý�ͷ�ιܼ�ˮ��ʹ��Һ��Һ��ǡ����̶������У�E.������ƿ�ǽ�����ҡ�ȡ�������B��A��C��F��D��E����3����û�н���C�����������ʵ����ʵ���ƫ�٣�ʹ��Һ��Ũ��ƫ�ͣ�������Һʱ������ƿδ������ڲ�Ӱ�����ʵ����ʵ�������Һ�������������Ƶ���Һ��Ũ����Ӱ�죻����ʱ���ӿ̶��ߣ�����Һ�����ƫ�٣�ʹ��Һ�����ʵ���Ũ��ƫ�ߡ�

 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д�