��Ŀ����
[���ʽṹ�����ʣ�13��]
��1����ͭͬ���ڡ���̬ԭ��������������ͬ�Ĺ���Ԫ�أ����̬ԭ�ӵĵ����Ų�ʽ ��
��2����ͼ���߱�ʾ���ֶ�����Ԫ�ص�ԭ��������������˳�����У����䳣�����ʷе�Ĺ�ϵ������A���ʾ�ĵ����� ���ѧʽ����
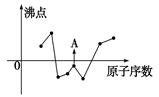
��3������������ӵĿռ乹���� �����廯�����Ȼ�����ӽṹ�������������ƣ������B��X�������������������������������ֵ��ʵ�����������ұ�����±����ʵ��ֵ�ȼ���ֵҪ�̵ö࣬���ܵ�ԭ���� ��
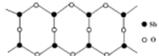
��4������Ʒ���Ӽ��������������Na+���������������ɵģ�ij�ֶ��������Ľṹ��ͼ��

����ԭ�ӵ��ӻ�����Ϊ ��
�����ֶ�������ƵĻ�ѧʽΪ ��
��5����֪HF��F��ͨ�������ϳ�HF ���ж�HF
���ж�HF ��HF
��HF �����ܷ��γ��������˵�����ɡ�
�����ܷ��γ��������˵�����ɡ�
��
��1����ͭͬ���ڡ���̬ԭ��������������ͬ�Ĺ���Ԫ�أ����̬ԭ�ӵĵ����Ų�ʽ ��
��2����ͼ���߱�ʾ���ֶ�����Ԫ�ص�ԭ��������������˳�����У����䳣�����ʷе�Ĺ�ϵ������A���ʾ�ĵ����� ���ѧʽ����

| ����/(pm) | B��F | B��Cl | B��Br |
| ����ֵ | 152 | 187 | 199 |
| ʵ��ֵ | 130 | 175 | 187 |
��4������Ʒ���Ӽ��������������Na+���������������ɵģ�ij�ֶ��������Ľṹ��ͼ��

����ԭ�ӵ��ӻ�����Ϊ ��
�����ֶ�������ƵĻ�ѧʽΪ ��
��5����֪HF��F��ͨ�������ϳ�HF
 ���ж�HF
���ж�HF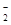 ��HF
��HF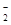 �����ܷ��γ��������˵�����ɡ�
�����ܷ��γ��������˵�����ɡ���
��1����Ar��3d54s1��2�֣� ��2��F2��2�֣�
��3��ƽ�������Σ�1�֣� B��Xԭ�Ӽ仹��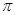 ���γɣ�2�֣�
���γɣ�2�֣�
��4��sp3��2�֣� Na5P3O10��2�֣�
��5����HF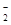 (F��H��F��)�У��Ѿ����ڷ��������������û�п������γ��������ԭ�ӡ���2�֣�
(F��H��F��)�У��Ѿ����ڷ��������������û�п������γ��������ԭ�ӡ���2�֣�
��3��ƽ�������Σ�1�֣� B��Xԭ�Ӽ仹��
 ���γɣ�2�֣�
���γɣ�2�֣���4��sp3��2�֣� Na5P3O10��2�֣�
��5����HF
 (F��H��F��)�У��Ѿ����ڷ��������������û�п������γ��������ԭ�ӡ���2�֣�
(F��H��F��)�У��Ѿ����ڷ��������������û�п������γ��������ԭ�ӡ���2�֣������������1����ͭͬ���ڡ���̬ԭ��������������ͬ�Ĺ���Ԫ�أ�Ϊ24��Ԫ��Cr�����̬ԭ�ӵĵ����Ų�ʽΪ��Ar��3d54s1����2��һ����˵������ķе����0�棬��������֪������ԭ�������ĵ�����������������Ԫ�صĵ���Ϊ���壬������ӦΪN��O��F��NeԪ�أ�����AӦΪF2����3�����������������ԭ����sp2�ӻ�������ԭ�ӳɼ�������������ӵĿռ乹����ƽ�������Σ���±����ʵ��ֵ�ȼ���ֵҪ�̵ö࣬���ܵ�ԭ������Bԭ������һ���յ�2p���������±��ԭ���ϵ��κ�һ���Ѿ��������ӵĶԳ�����ͬ��p�������һ�Ե����γ��˦м�����4������ԭ���γ����ĸ��Ҽ���û�йµ��Ӷԣ��۵��Ӷ���Ϊ4���ʳ�sp3�ӻ������ɸ����Ķ��������ṹʽ֪������3�������������ӣ��൱����3�������������ȥ���ˣ�3-1����ԭ�ӣ��������Ϊ-2����3��3+1��+5��3=-��3+2�����ɻ��ϼ۹���֪�����Ƶ����ΪNa5P3O10����5����HF(F��H��F��)�У��Ѿ����ڷ��������������û�п������γ��������ԭ�ӡ�

��ϰ��ϵ�д�
�����Ŀ