��Ŀ����
��.�ִ���ҵ�����Ȼ���Ϊԭ���Ʊ�������ֹ����������£�
 k+s-5#
k+s-5#
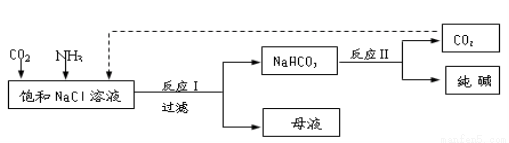
��֪NaHCO3�ڵ������ܽ�Ƚ�С����Ӧ��Ϊ��NaCl+CO2+NH3+H2O NaHCO3��+NH4Cl������ĸҺ�����ַ������£�
NaHCO3��+NH4Cl������ĸҺ�����ַ������£�
��1����ĸҺ�м���ʯ���飬�ɽ�����________ѭ�����á�
��2����ĸҺ��ͨ��NH3������ϸС��ʳ�ο��������£��ɵõ�NH4Cl���塣��д��ͨ��NH3���ܽ�Ƚ�С����ʽ̼����ת��Ϊ�ܽ�Ƚϴ��̼���ε����ӷ���ʽ _____________________________��
��.ij��ѧС��ģ���������Ƽ������NaCl��NH3��CO2��ˮ��Ϊԭ���Լ���ͼ��ʾװ����ȡNaHCO3��Ȼ���ٽ�NaHCO3�Ƴ�Na2CO3��
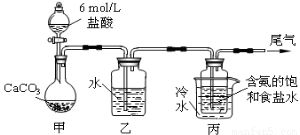
��1��װ�ñ�����ˮ�������� ��
��2����װ�ñ��в�����NaHCO3��ȡNa2CO3ʱ����Ҫ���е�ʵ�������_______��ϴ�ӡ����ա�NaHCO3ת��ΪNa2CO3�Ļ�ѧ����ʽΪ ��
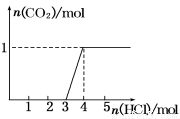
��3�����ڣ�2�������յ�ʱ��϶̣�NaHCO3���ֽⲻ��ȫ����С���һ�ݼ�����t1 min��NaHCO3 ��Ʒ����ɽ���������̽����ȡ������t1 min��NaHCO3��Ʒ29.6 g��ȫ����ˮ�Ƴ���Һ��Ȼ�������Һ�л����صμ�ϡ���ᣬ�����Ͻ��衣��������ļ��룬��Һ���й����ӵ����ʵ����ı仯��ͼ��ʾ��������a��Ӧ����Һ�е�������___________�������ӷ�����ͬ��������c��Ӧ����Һ�е�������___________������Ʒ��NaHCO3��Na2CO3�����ʵ���֮���� ��
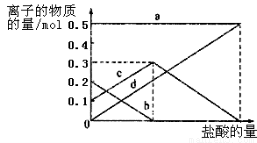
��4����ȡ21.0 g NaHCO3���壬������t2 rnin��ʣ����������Ϊl4.8 g������Ѵ�ʣ�����ȫ�����뵽200 mL 2 mol?L��1�������У����ַ�Ӧ����Һ��H+ �����ʵ���Ũ��Ϊ____________������Һ����仯���Բ��ƣ�
����1��NH3 ��2�֣�
��2��HCO3�C+NH3=NH4++CO32�C��2�֣�
��.
��1����ȴ��ʹ̼�����ƾ���������1�֣�
��2�����ˣ�1�֣� 2NaHCO3 Na2CO3+H2O+CO2����2�֣�
Na2CO3+H2O+CO2����2�֣�
��3��Na+ ��1�֣� HCO3- ��1�֣� 1:2 ��2�֣�
��4��0.75 mol/L ��2�֣�
��������
�������������1������ʯ�����Ca(OH)2��NH4Cl��Ӧ����NH3������NH3��ѭ��ʹ�á�
��2���ܽ�Ƚ�С����ʽ̼����ת��Ϊ�ܽ�Ƚϴ��̼���Σ�NaHCO3�е�CO32?��NH3��Ӧ����NH4+��CO32?���ɵ����ӷ���ʽ��
����1������ʱNaHCO3���ܽ�ȼ�С������װ�ñ�����ˮ��������װ�ñ�����ˮ�������ǣ���ȴ��ʹ̼�����ƾ���������
��2��ͨ�����˵ĵ�NaHCO3���壻NaHCO3����ֽ�õ�Na2CO3��CO2��H2O����д�ɻ�ѧ����ʽ��
��3����������ļ��룬������Ӧ CO32-+H+=HCO3-�� HCO3-+H+=CO2��+H2O����Һ���й����ӵ����ʵ����ı仯Ϊ̼������Ӽ�С��̼���������Ũ������̼�������ȫ��ת��Ϊ̼��������ӣ��ٵ��������̼��������ӷ�Ӧ���ɶ�����̼��̼��������Ӽ�С������c���߱�ʾ����̼���������Ũ�ȱ仯��Na+Ũ�Ȳ��䣬����a���߱�ʾNa+��Ũ�ȱ仯��̼�������Ũ��0.2mol/L��̼���������Ũ��Ϊ0.1mol/L����Ʒ��NaHCO3��Na2CO3�����ʵ���֮����1��2��
��4��NaHCO3���ȷֽ�����Na2CO3���ɻ�ѧ����ʽ��֪�������ᷴӦ���ĵ�HCl������ͬ�����Է�Ӧ��H+Ũ��Ϊc(H+)=(0.2L��2mol?L?1-21g��84g/mol)��0.2L= 0.75 mol?L?1��
���㣺���⿼����ҵ�ƴ����ԭ�����������������е����ʱ仯�������ɷֵķ����жϺͼ���Ӧ�ã�ʵ����̷��������Ӳ�����β�����գ�ͼ���������жϡ�

 ��У����ϵ�д�
��У����ϵ�д�