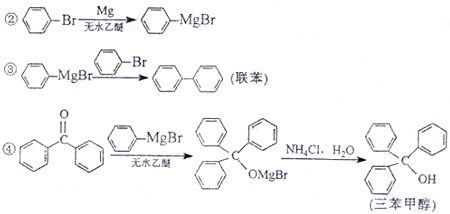
��Ŀ����
��ͼ1��ʾ����30ml 0.1mol?L-1Ba��OH��2��Һ�����ձ��У�Ȼ����������0.1mol?L-1ij��������Ԫ���γɵ�ij����Һ��25mL����������Һ�����V�����ǿ��I��I-Vͼ��ͼ2��ʾ����1������Ļ�ѧʽΪ______��
��2������C��ʱ��Һ�е������������Ҫ��______��
��3������F��ʱ����Һ�е������������Ҫ��______��
��4���ձ��иռ�����ʱ���۲쵽��������______��
��5����A��E����ǿ��I��С����Ҫԭ����______��

���𰸡���������1��n��Ba��OH��2��=0.03L×0.1mol/L=0.003mol��n���ᣩ=0.1moL/L×0.02L=0.002mol������ж�ӦΪ��Ԫ�ᣬ�����
��2����C�㣨������������Ϊ10mL��ʱ���ձ��е�Ba��OH��2��û�б���ȫ�кͣ�
��3��Fʱ�����������Һ�д��ڵ���������H2PO4-��
��4���ձ��иռ�����ʱ��1Ba��OH��2���������ɳ�����
��5������������ˮ��Ba3��PO4��2���ѵ����ˮ������Ũ�Ƚ��ͣ�
����⣺��1���ڵ�3����Ԫ���У����γɵ�������ǹ��ᣨH2SiO3��������ˮ�������ᣨH3PO4����ǿ�ᣩ�����ᣨH2SO4����Ԫǿ�ᣩ�������ᣨHClO4�������ᣨHClO3���������ᣨHClO�������ᣨHCl����������֪�������ɵ�3����ijԪ���γɵ�ij����Һ������֪����϶����ǹ��ᣨ��Ϊ������ˮ�������������ᡢ���ᣬ���Ǹ������һԪ�ᣬ����Ҫ����һ���ط��������и������ᡢ���Ũ�Ⱥ������������ǡ����ȫ�к�ʱ����Ӧ��������ͼ2��E��ĺ�������ֵ������ɣ�����������˵�����кͷ�Ӧ�Ĺ�ϵʽ��H2SO4��Ba��OH��2��ǡ����ȫ�к�ʱ����Ӧ�������������ӦΪ30mL����ͼ2��E��ĺ�����ֵ20������˵����������ᣮͬ�������������ó�����Ǹ������һԪ�ᣬֻ�������ᣮ�������֤���̣������кͷ�Ӧ�Ĺ�ϵʽΪ2H3PO4��3Ba��OH��2���ó���Ҫ��������Ϊ20mL����ͼ2��E��ĺ�������������ԣ�������H3PO4���ʴ�Ϊ��H3PO4��
��2����C�㣨������������Ϊ10mL��ʱ���ձ��е�Ba��OH��2��û�б���ȫ�кͣ���Һ�е������������Ҫ��Ba2+���ʴ�Ϊ��Ba2+��
��3��Fʱ��������������������H2PO4-���ʴ�Ϊ��H2PO4-��
��4�����ձ��иռ�������ʱ�������ķ�Ӧ�Ļ�ѧ����ʽΪ2H3PO4+3Ba��OH��2=Ba3��PO4��2��+6H2O�����Թ۲쵽����������Һ�в�����ɫ�������ʴ�Ϊ���а�ɫ�������ɣ�
��5��ͼ2�У���A��E����ǿ��I��С����Ҫԭ���������кͷ�Ӧ�ķ���������������ˮ��Ba3��PO4��2���ѵ����ˮ��ʹ��Һ�������ƶ�������Ũ���½���
�ʴ�Ϊ���������ܵ�Ba3��PO4��2���ѵ����ˮ��ʹ��Һ�е�����Ũ�Ƚ��ͣ�
���������⿼����ԭ������Ŀ����������ϵļ�����жϣ���Ŀ�Ѷ��еȣ�ע����ղμӷ�Ӧ�����ʵ����Ĺ�ϵ��Ϊ������Ĺؼ���
��2����C�㣨������������Ϊ10mL��ʱ���ձ��е�Ba��OH��2��û�б���ȫ�кͣ�
��3��Fʱ�����������Һ�д��ڵ���������H2PO4-��
��4���ձ��иռ�����ʱ��1Ba��OH��2���������ɳ�����
��5������������ˮ��Ba3��PO4��2���ѵ����ˮ������Ũ�Ƚ��ͣ�
����⣺��1���ڵ�3����Ԫ���У����γɵ�������ǹ��ᣨH2SiO3��������ˮ�������ᣨH3PO4����ǿ�ᣩ�����ᣨH2SO4����Ԫǿ�ᣩ�������ᣨHClO4�������ᣨHClO3���������ᣨHClO�������ᣨHCl����������֪�������ɵ�3����ijԪ���γɵ�ij����Һ������֪����϶����ǹ��ᣨ��Ϊ������ˮ�������������ᡢ���ᣬ���Ǹ������һԪ�ᣬ����Ҫ����һ���ط��������и������ᡢ���Ũ�Ⱥ������������ǡ����ȫ�к�ʱ����Ӧ��������ͼ2��E��ĺ�������ֵ������ɣ�����������˵�����кͷ�Ӧ�Ĺ�ϵʽ��H2SO4��Ba��OH��2��ǡ����ȫ�к�ʱ����Ӧ�������������ӦΪ30mL����ͼ2��E��ĺ�����ֵ20������˵����������ᣮͬ�������������ó�����Ǹ������һԪ�ᣬֻ�������ᣮ�������֤���̣������кͷ�Ӧ�Ĺ�ϵʽΪ2H3PO4��3Ba��OH��2���ó���Ҫ��������Ϊ20mL����ͼ2��E��ĺ�������������ԣ�������H3PO4���ʴ�Ϊ��H3PO4��
��2����C�㣨������������Ϊ10mL��ʱ���ձ��е�Ba��OH��2��û�б���ȫ�кͣ���Һ�е������������Ҫ��Ba2+���ʴ�Ϊ��Ba2+��
��3��Fʱ��������������������H2PO4-���ʴ�Ϊ��H2PO4-��
��4�����ձ��иռ�������ʱ�������ķ�Ӧ�Ļ�ѧ����ʽΪ2H3PO4+3Ba��OH��2=Ba3��PO4��2��+6H2O�����Թ۲쵽����������Һ�в�����ɫ�������ʴ�Ϊ���а�ɫ�������ɣ�
��5��ͼ2�У���A��E����ǿ��I��С����Ҫԭ���������кͷ�Ӧ�ķ���������������ˮ��Ba3��PO4��2���ѵ����ˮ��ʹ��Һ�������ƶ�������Ũ���½���
�ʴ�Ϊ���������ܵ�Ba3��PO4��2���ѵ����ˮ��ʹ��Һ�е�����Ũ�Ƚ��ͣ�
���������⿼����ԭ������Ŀ����������ϵļ�����жϣ���Ŀ�Ѷ��еȣ�ע����ղμӷ�Ӧ�����ʵ����Ĺ�ϵ��Ϊ������Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
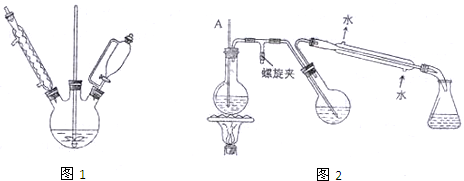
Ӳ�ʲ������ǻ�ѧʵ���о���ʹ�õ�һ����������������ʵ�飨�̶�װ���ԣ����ش�
��1��������ʵ�飺��ͼ1��ʾ����Ũ�������װ��Na2SO3�����������һ��ʱ���a��b��c��������仯���±���
��2����ͼ2��ʾ����Ӳ�ʲ����ܸ�װΪȼ�չܣ���ѹ����ƿ�ڣ���װ�й���B����ƿ�м�����ҺA��ͬʱ��ȼ�չ���ͨ������C����ȼ���ɿ������Ե�ȼ�������������Է�ֹ�����ϱ�ը����
���ô�װ��ģ�ҵ�ϳ����ᣬ������Լ�ѡ����ȷ���� ������ţ����ܿڿɹ۲쵽 ɫ���森
������ҺAΪ����ʳ��ˮ������CΪ������ȼ�չ��ڹ۲쵽����������ŨŨ���̣������BΪ ���ѧʽ�����˻����¶ȼ��ߣ����� ���������и�ӽ�����

��1��������ʵ�飺��ͼ1��ʾ����Ũ�������װ��Na2SO3�����������һ��ʱ���a��b��c��������仯���±���
| ���� | �����ϵμ��Լ� | ʵ������ | ���ͻ���� |
| a | �����ף� �Ⱥ��ָֻ���ɫ |
���ͣ� ���ۣ����������Ư���ԣ� | |
| b | ����̪��NaOH��Һ | �����Ϊ��ɫ | ���ӷ���ʽ�� |
| c | �����Ϊ��ɫ | ��ѧ����ʽ�� ���ۣ���������� |
���ô�װ��ģ�ҵ�ϳ����ᣬ������Լ�ѡ����ȷ����
������ҺAΪ����ʳ��ˮ������CΪ������ȼ�չ��ڹ۲쵽����������ŨŨ���̣������BΪ
| ��ҺA | ����B | ����C | |
| a | ϡ���� | Zn | Cl2 |
| b | Ũ���� | MnO2 | H2 |
| c | ϡ���� | Fe | Cl2 |
| d | Ũ���� | KMnO4 | H2 |
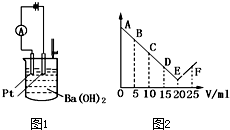 ��ͼ1��ʾ����30ml 0.1mol?L-1Ba��OH��2��Һ�����ձ��У�Ȼ����������0.1mol?L-1ij��������Ԫ���γɵ�ij����Һ��25mL����������Һ�����V�����ǿ��I��I-Vͼ��ͼ2��ʾ��
��ͼ1��ʾ����30ml 0.1mol?L-1Ba��OH��2��Һ�����ձ��У�Ȼ����������0.1mol?L-1ij��������Ԫ���γɵ�ij����Һ��25mL����������Һ�����V�����ǿ��I��I-Vͼ��ͼ2��ʾ��
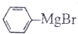 �������ͪ��Ӧ�Ʊ������״���
�������ͪ��Ӧ�Ʊ������״���