��Ŀ����
18.0 mol��L-1��Ũ����ϡ�ͳ�2.00 mol��L-1��ϡ�� ��100 ml,ʵ��������£�
��1�����㲢����Ͳ��ȡ ml��18.0 mol��L-1��Ũ����;
��2���� ���ձ��ڱ�����ע��ʢ������ ���ձ��У�
��3��������ȴ�����µ�������Һ�ز�����ע�� ___________��
��4������������ˮϴ���ձ�2~3��,����ϴ��ҺҲȫ��ת�Ƶ�����ƿ�У�
��5������������ƿ�м�����ˮ,ֱ��Һ��ӽ��̶���1~2cm�������ý�ͷ�ι���μ�����ˮ����Һ����̶�������
��6���Ǻ�����ƿ���������ߵ�ҡ�ȣ�����õ�ϡ���ᵹ���Լ�ƿ�У����ñ�ǩ���档
�Է������в�����ʵ������Ӱ�죨ƫ�ߡ�ƫ�ͻ���Ӱ�죩
��û��ϴ���ձ��Ͳ�����
�ڶ���ʱ���Ӷ���
������ƿʹ��ǰ������ˮ����
��1�����㲢����Ͳ��ȡ ml��18.0 mol��L-1��Ũ����;
��2���� ���ձ��ڱ�����ע��ʢ������ ���ձ��У�
��3��������ȴ�����µ�������Һ�ز�����ע�� ___________��
��4������������ˮϴ���ձ�2~3��,����ϴ��ҺҲȫ��ת�Ƶ�����ƿ�У�
��5������������ƿ�м�����ˮ,ֱ��Һ��ӽ��̶���1~2cm�������ý�ͷ�ι���μ�����ˮ����Һ����̶�������
��6���Ǻ�����ƿ���������ߵ�ҡ�ȣ�����õ�ϡ���ᵹ���Լ�ƿ�У����ñ�ǩ���档
�Է������в�����ʵ������Ӱ�죨ƫ�ߡ�ƫ�ͻ���Ӱ�죩
��û��ϴ���ձ��Ͳ�����
�ڶ���ʱ���Ӷ���
������ƿʹ��ǰ������ˮ����
��1�� 11.1 ��2�� Ũ���� �� ˮ
��3�� 100mL����ƿ ��6���� ƫ�� �� ƫ�� �� ��Ӱ��
��3�� 100mL����ƿ ��6���� ƫ�� �� ƫ�� �� ��Ӱ��
��1��ϡ���������ʲ��䣬����Ũ����������
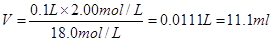 ��
��
��2��Ũ��������ˮ�ų��������ȣ���Ũ������ܶȴ���ˮ�ġ�����ϡ��Ũ����Ӧ���ǽ�Ũ�������ձ��ڱ�����ע��ʢ������ˮ���ձ��С�
��3��ȷ����һ�����ʵ���Ũ����Һʱ����Ҫ����Ӧ��������ƿ��
��6����������Ҫʱ����c��n/V��
��û��ϴ�ӣ�������ƫ�٣�Ũ��ƫ�͡�
�ڶ���ʱ���Ӷ�����������ƿ����Һ�����ƫ�٣�Ũ��ƫ�ߡ�
��������Ҫ������ˮ���ݣ���������ƿ�к�������ˮ�Խ���Dz�Ӱ��ġ�
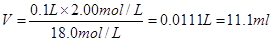 ��
����2��Ũ��������ˮ�ų��������ȣ���Ũ������ܶȴ���ˮ�ġ�����ϡ��Ũ����Ӧ���ǽ�Ũ�������ձ��ڱ�����ע��ʢ������ˮ���ձ��С�
��3��ȷ����һ�����ʵ���Ũ����Һʱ����Ҫ����Ӧ��������ƿ��
��6����������Ҫʱ����c��n/V��
��û��ϴ�ӣ�������ƫ�٣�Ũ��ƫ�͡�
�ڶ���ʱ���Ӷ�����������ƿ����Һ�����ƫ�٣�Ũ��ƫ�ߡ�
��������Ҫ������ˮ���ݣ���������ƿ�к�������ˮ�Խ���Dz�Ӱ��ġ�

��ϰ��ϵ�д�
�����Ŀ

