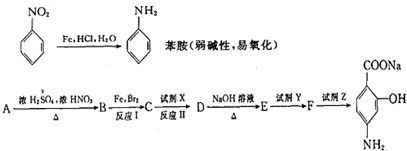
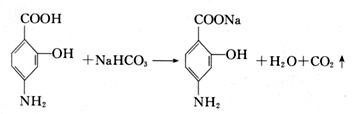
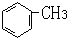��Ŀ����
��2011?�����ģ������ͭ���ȷֽ���������ͭ�����壬�����¶Ȳ�ͬ��������ɷ�Ҳ��ͬ������ɷֿ��ܺ�SO2��SO3��O2�е�һ�֡����ֻ����֣�ij��ѧ����С��ͨ�����һ̽����ʵ�飬�ⶨ��Ӧ������SO2��SO3��O2�����ʵ�������������ȷ�������ʵĻ�ѧ���������Ӷ�ȷ��CuSO4�ֽ�Ļ�ѧ����ʽ��ʵ���õ�����������ͼ��ʾ��

[�������]
����������ijɷֿ���ֻ��
����������ijɷֿ��ܺ���
����������ijɷֿ��ܺ���SO2��SO3��O2���֣�
[ʵ��̽��]
ʵ��������̣��ԣ�����֪ʵ�����ʱ������ͭ��ȫ�ֽ⣮
��1��������װ̽��ʵ���װ�ã����������ҵķ��������ӿ�����˳��Ϊ
��2��ʵ������У�����C��������
[��֤���裬��������]
��3����ʵ�������B����Ͳû���ռ���ˮ����֤������
��4��������ʵ��С����и�ʵ�飬���ڼ���ʱ���¶Ȳ�ͬ��ʵ����������������Ҳ��ͬ���������£�
��ͨ�����㣬�ƶϳ��ڢ�С��͵ڢ�С���ʵ��������CuSO4�ֽ�Ļ�ѧ��Ӧ����ʽ��
����

[�������]
����������ijɷֿ���ֻ��
SO3
SO3
һ�֣�����������ijɷֿ��ܺ���
SO2��O2
SO2��O2
���֣�����������ijɷֿ��ܺ���SO2��SO3��O2���֣�
[ʵ��̽��]
ʵ��������̣��ԣ�����֪ʵ�����ʱ������ͭ��ȫ�ֽ⣮
��1��������װ̽��ʵ���װ�ã����������ҵķ��������ӿ�����˳��Ϊ
�٢ۢܢޢݢ�
�٢ۢܢޢݢ�
������ţ�����2��ʵ������У�����C��������
����SO2��SO3����
����SO2��SO3����
��[��֤���裬��������]
��3����ʵ�������B����Ͳû���ռ���ˮ����֤������
I
I
��ȷ����4��������ʵ��С����и�ʵ�飬���ڼ���ʱ���¶Ȳ�ͬ��ʵ����������������Ҳ��ͬ���������£�
| ʵ�� С�� |
��ȡCuSO4 ������/g |
����C�� �ӵ�����/g |
��Ͳ��ˮ���������ɱ� ״������������/mL |
| �� | 6.4 | 2.88 | 224 |
| �� | 6.4 | 2.56 | 448 |
����
4CuSO4
4CuO+2SO3��+2SO2��+O2��
| ||
4CuSO4
4CuO+2SO3��+2SO2��+O2��
������
| ||
2CuSO4
2CuO+2SO2��+O2��
| ||
2CuSO4
2CuO+2SO2��+O2��
��
| ||
��������1����������ͭ�ֽ���ܷ����������2CuSO4
2CuO+2SO2��+O2���� CuSO4
CuO+SO3��������������Ӧͬʱ�������жϣ�
��2������������Ʊ����ռ����շ���װ�á���������װ�á���ˮ������װ������װʵ��������
��3��B����Ͳû���ռ���ˮ����˵����Ӧû���������ɣ�
��4���ⶨ��Ӧ������SO2��SO3��O2�����ʵ�������������ȷ�������ʵĻ�ѧ���������Ӷ�ȷ��CuSO4�ֽ�Ļ�ѧ����ʽ��
| ||
| ||
��2������������Ʊ����ռ����շ���װ�á���������װ�á���ˮ������װ������װʵ��������
��3��B����Ͳû���ռ���ˮ����˵����Ӧû���������ɣ�
��4���ⶨ��Ӧ������SO2��SO3��O2�����ʵ�������������ȷ�������ʵĻ�ѧ���������Ӷ�ȷ��CuSO4�ֽ�Ļ�ѧ����ʽ��
����⣺��������ͭ�ֽ���ܷ����������2CuSO4
2CuO+2SO2��+O2���� CuSO4
CuO+SO3��������������Ӧͬʱ�������жϣ��ʴ�Ϊ��SO3��SO2��O2��
��1��������Ʊ����ռ����շ���װ�á���������װ�á���ˮ������װ������װʵ���������ʴ�Ϊ���٢ۢܢޢݢڣ�
��2��SO2��SO3��������������ʷ�Ӧ���ü�ʯ�ҿ�����SO2��SO3���壬�ʴ�Ϊ������SO2��SO3���壻
��3��B����Ͳû���ռ���ˮ����˵����Ӧû���������ɣ��ʴ�Ϊ��I��
��4������6.4g����ͭ�ֽ�����xSO3molSO3��ymolSO2������
��֮�ã�x=0.02mol��y=0.02mol��
��n��O2��=
=0.01mol���������ߵ����ʵ���֮��Ϊ2��2��1���ʴ�Ϊ��4CuSO4
4CuO+2SO3��+2SO2��+O2����
����6.4g����ͭ�ֽ�����mmolSO3��nmolSO2������
����֮�ã�m=0��n=0.04mol����n��O2��=
=0.02mol�����Զ��ߵ����ʵ���֮��Ϊ2��1��
�ʴ�Ϊ��2CuSO4
2CuO+2SO2��+O2����
| ||
| ||
��1��������Ʊ����ռ����շ���װ�á���������װ�á���ˮ������װ������װʵ���������ʴ�Ϊ���٢ۢܢޢݢڣ�
��2��SO2��SO3��������������ʷ�Ӧ���ü�ʯ�ҿ�����SO2��SO3���壬�ʴ�Ϊ������SO2��SO3���壻
��3��B����Ͳû���ռ���ˮ����˵����Ӧû���������ɣ��ʴ�Ϊ��I��
��4������6.4g����ͭ�ֽ�����xSO3molSO3��ymolSO2������
|
��n��O2��=
| 224��10-3 |
| 22.4 |
| ||
����6.4g����ͭ�ֽ�����mmolSO3��nmolSO2������
|
| 448��10-3 |
| 22.4 |
�ʴ�Ϊ��2CuSO4
| ||
���������⿼�����ʵ���ɺ�ʵ�����ݵĴ���������ʱע�����ʵ�����֪ʶ����������й����ݽ��м��㣬�������һ���Ѷȣ�

��ϰ��ϵ�д�
�����Ŀ
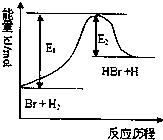 ��2011?�����ģ�����շ�ӦBr+H2?HBr+H�������Է�Ӧ���̵�ʾ��ͼ��������������ȷ���ǣ�������
��2011?�����ģ�����շ�ӦBr+H2?HBr+H�������Է�Ӧ���̵�ʾ��ͼ��������������ȷ���ǣ������� ��ƻ������ܷ����ķ�Ӧ�ǣ�������
��ƻ������ܷ����ķ�Ӧ�ǣ������� ��2011?�����ģ��A��B��C��D��E����Ԫ�ض���36����ǰԪ�أ�ԭ���������������������Ϣ���±�
��2011?�����ģ��A��B��C��D��E����Ԫ�ض���36����ǰԪ�أ�ԭ���������������������Ϣ���±�