��Ŀ����
537�桢1.01��105Pa ʱ�����ݻ��ɱ���ܱ������г���2mol SO2�� 1mol O2����ʱ���������Ϊ200L���������м�����������壩�����ֺ��º�ѹ��������Ӧ��
2SO2������+O2������![]() 2SO3������
2SO3������
�ﵽƽ��ʱ��ƽ��������SO3���������Ϊ0.91��
�Իش��������⣺
��1����ҵ�϶�������Ĵ��������ó�ѹ�������ø�ѹ��ԭ���ǣ� _______��
��2�����������¶Ⱥ�ѹǿ���䣬����������ֻ����2mol SO3����������������ƽ��ʱ��SO2����������� �����������Ϊ L��
��3���¶��Ա���537�棬�����������200L���䣨���ݣ�������a molSO2��b molO2������������������Ӧ��ƽ��ʱ��SO3�����������Ϊ0.91����ϵѹǿΪ1.01��105Pa����a:b=2:1����a= ��
��1����ѹ�£���������ĺ����Ѵﵽ91%���ӽ��ͳɱ����ǣ�û�б�Ҫ�ټ�ѹ��
��2 ��6%��137.5L��
��3��2.9��
������2molSO3������2molSO2��1molO2�൱��������������ͬʱ���Դﵽ��ͬ��ƽ��״̬������ƽ��ʱ������������������6%���������������Ϊ3%����ƽ��ʱSO3�����ʵ���Ϊxmol������![]() =0.91�������x=1.88��ƽ��ʱ������ܵ����ʵ���Ϊ2.062mol������ƽ��ʱ����������ΪV=
=0.91�������x=1.88��ƽ��ʱ������ܵ����ʵ���Ϊ2.062mol������ƽ��ʱ����������ΪV=![]() =137.5L����3���У����ʼ�ձ���200L���ﵽƽ��ʱ�����Ҳ��200L������������������ʵ���Ϊ
=137.5L����3���У����ʼ�ձ���200L���ﵽƽ��ʱ�����Ҳ��200L������������������ʵ���Ϊ![]() =2.9mol����
=2.9mol����

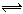 2Z(g)���ﵽƽ��ʱ��ƽ����������Z���������Ϊ0.5��
2Z(g)���ﵽƽ��ʱ��ƽ����������Z���������Ϊ0.5��