��Ŀ����
һ�������£�ͨ�����з�Ӧ��ʵ��ȼú�����е���Ļ��գ�SO2(g)��2CO(g) 2CO2(g)��S(l) ��H��0��һ���¶��£����ݻ�Ϊ2L�ĺ����ܱ�������1molSO2��nmolCO������Ӧ��5min��ﵽƽ�⣬����2amolCO2������˵����ȷ����
2CO2(g)��S(l) ��H��0��һ���¶��£����ݻ�Ϊ2L�ĺ����ܱ�������1molSO2��nmolCO������Ӧ��5min��ﵽƽ�⣬����2amolCO2������˵����ȷ����
| A����Ӧǰ2min��ƽ������v(SO2)��0.1amol/(L.min) |
| B���������������ʵ������ٸı�ʱ����Ӧ�ﵽƽ��״̬ |
| C��ƽ��������������䣬�������з����������ƽ��������Ӧ�����ƶ� |
| D��ƽ��������������䣬�����¶Ⱥͼ��������SO2��ת���ʾ����� |
B
�������������A�����ݷ���ʽ��֪������2amolCO2��ͬʱ������amolSO2����Ũ����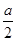 mol/L�����Է�Ӧǰ2min��ƽ������v(SO2)��
mol/L�����Է�Ӧǰ2min��ƽ������v(SO2)��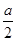 mol/L ��2min��0.25amol/(L��min)��A����ȷ��B�����ݷ���ʽ��֪���÷�Ӧ����������ʵ�����С�Ŀ��淴Ӧ����˵������������ʵ������ٸı�ʱ������˵����Ӧ�ﵽƽ��״̬��B��ȷ��C��S�ǹ��壬�ı�����������ƽ�ⲻ�ƶ���C����ȷ��D���÷�Ӧ�Ƿ��ȷ�Ӧ�������¶�ƽ�����淴Ӧ�����ƶ���SO2��ת���ʽ��͡��������ܸı�ƽ��״̬��ת���ʲ��䣬D����ȷ����ѡB��
mol/L ��2min��0.25amol/(L��min)��A����ȷ��B�����ݷ���ʽ��֪���÷�Ӧ����������ʵ�����С�Ŀ��淴Ӧ����˵������������ʵ������ٸı�ʱ������˵����Ӧ�ﵽƽ��״̬��B��ȷ��C��S�ǹ��壬�ı�����������ƽ�ⲻ�ƶ���C����ȷ��D���÷�Ӧ�Ƿ��ȷ�Ӧ�������¶�ƽ�����淴Ӧ�����ƶ���SO2��ת���ʽ��͡��������ܸı�ƽ��״̬��ת���ʲ��䣬D����ȷ����ѡB��
���㣺���鷴Ӧ���ʵļ��㡢���������ƽ��״̬��Ӱ���Լ�ƽ��״̬���ж�

��ӦP(g)+Q(g) M(g)+N(s) ��H��0���ﵽƽ��ʱ������˵����ȷ����
M(g)+N(s) ��H��0���ﵽƽ��ʱ������˵����ȷ����
| A����С���������ƽ�ⲻ�ƶ� | B�����������M�IJ������� |
| C������c(P)��P��ת�������� | D�������¶ȣ�Q��ת�������� |
��Ӧ3X(g)+Y(g)  2Z(g)+2W(g)��2L�ܱ������н��У�5min��Y������0.5mol����˷�Ӧ������vΪ( )
2Z(g)+2W(g)��2L�ܱ������н��У�5min��Y������0.5mol����˷�Ӧ������vΪ( )
| A��v(X)=0.05mol��L�C1��min�C1 | B��v(Z)=0.10mol��L�C1��min�C1 |
| C��v(Y)=0.10mol��L�C1��min�C1 | D��v(W)=0.05mol��L�C1��min�C1 |
ij�ݻ��ɱ���ܱ������г�������ʵ���������A��B��һ���¶��·�����Ӧ��A(g)+B(g) 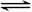 2C(g)+Q��Q>0��������������Ũ����ʱ��仯��������ͼ��ʾ������˵������ȷ����
2C(g)+Q��Q>0��������������Ũ����ʱ��仯��������ͼ��ʾ������˵������ȷ����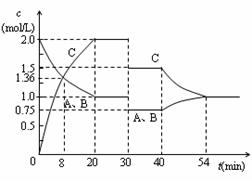
| A��30minʱ�����¶ȣ�40minʱ�����¶� |
| B��40min��54min֮�䷴Ӧ����v(��) < v(��) |
| C����20min��30minʱ��ƽ�ⳣ���ֱ�ΪK1��K2����K1 >K2 |
| D��0~8minA��ƽ����Ӧ����Ϊ0��64mol/(L��min) |
��֪��Ӧ2CH3OH(g) CH3OCH3(g)+H2O(g)��ij�¶��µ�ƽ�ⳣ��Ϊ400�����¶��£�������㶨���ܱ������м���һ������CH3OH����Ӧ��ijʱ�̲�ø���ֵ�Ũ�����£�
CH3OCH3(g)+H2O(g)��ij�¶��µ�ƽ�ⳣ��Ϊ400�����¶��£�������㶨���ܱ������м���һ������CH3OH����Ӧ��ijʱ�̲�ø���ֵ�Ũ�����£�
| ���� | CH3OH | CH3OCH3 | H2O |
| Ũ��/mol��L-1 | 0.44 | 0.6 | 0.6 |
������������ȷ����
A���÷�Ӧ��ƽ�ⳣ������ʽΪK��[c(CH3OCH3)��c(H2O)]/c(CH3OH)
B����ʱ�����淴Ӧ���ʵĴ�С������������
C������10min��Ӧ�ﵽƽ�⣬��ʱc(CH3OH)��0.04mol/( L��min)
D��0��10min��ƽ����Ӧ���ʦ�(CH3OH)��1.6mol/(L��min)
һ�������£��ֱ����ݻ��̶����ܱ������г���A������B��������Ӧ���£�2A(g)+B(s) 2D(g)��H��0,�������������£�������֪����˵������ȷ����
2D(g)��H��0,�������������£�������֪����˵������ȷ����
| | ʵ��� | ʵ��� | ʵ��� |
| ��Ӧ�¶�/�� | 800 | 800 | 850 |
| c(A)��ʼ/mol��L��1 | 1 | 2 | 1 |
| c(A)ƽ��/mol��L��1 | .0.5 | 1 | 0.85 |
| �ų�������/kJ | a | b | c |
A.ʵ��III�Ļ�ѧƽ�ⳣ��K��1
B.ʵ��ų���������ϵΪb��2a
C.ʵ��III��30min�ﵽƽ��ʱ������v(A)Ϊ0.005mol? L-1��min-1
D.�������������ܶȲ���ʱ����仯ʱ������Ӧ�ﵽƽ��
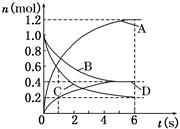
 6A��2D
6A��2D
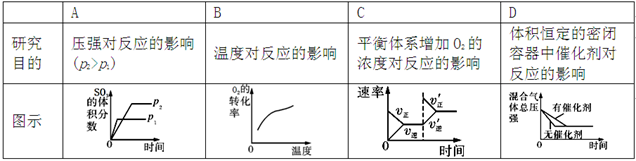
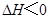 �������о�Ŀ�ĺ�ʾ��ͼ�������
�������о�Ŀ�ĺ�ʾ��ͼ�������