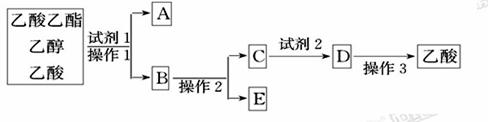
��Ŀ����
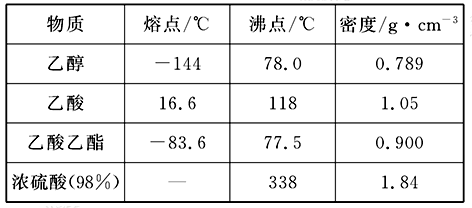
������Ӧ�Ǽ���ȩ������Ҫ��Ӧ���̲ĶԸ�ʵ��IJ�������ֻ�Ǵ��Ե�������ijͬѧ���������о���
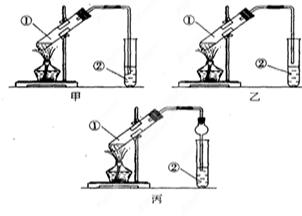
��1���ڸɾ��Թ��м���2ml2�� ��Ȼ�� �õ�������Һ����װ��5֧�Թܣ����Ϊ1#��2#��3#��4#��5# ��
��2�����εμ�2%��5%��10%��20%��40%����ȩ��Һ4�Σ�����������60��~70���ˮԡ�С�3���Ӻ��Թ�1#δ�γɴ�����������Թ�5# �������������кڰߣ� �Թ�4# ��������������һ�㣬�Թ�2#��3# �γɹ�����������
���о���Ŀ���ǣ� ��
��3����ȩ����������Ӧ�Ļ�ѧ����ʽΪ�� ��
��4����������:��ǿ���������£�����������Һ����������������֤�ͶԱ�ʵ�����¡�
��ͬѧ������ʵ����ʵ���IJ��죬�������Ϻ�֪:
a��Ag(NH3)2++2H2O Ag++2NH3��H2O�� b��AgOH���ȶ������ֽ�Ϊ��ɫAg2O
Ag++2NH3��H2O�� b��AgOH���ȶ������ֽ�Ϊ��ɫAg2O
�����飬ʵ������������NH3,��ɫ��������Ag2O������Ag2O��ԭ���ǣ�
�������������������� ��
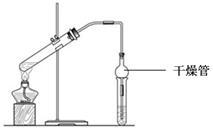
��5����ʪ��ĺ�ɫʯ����ֽ����NH3�������������������������������������������� ����ϡHNO3�� ϴ�Թܱ��ϵ�Ag���÷�Ӧ�Ļ�ѧ����ʽ������������������ �� �������� ��
��6����ͬѧ�Բ���������ԭ���������:��NaOH�����£�������NH3��ԭAg2O������ü����������˵������ȩ��ʱ��������Һ���ܳ�ǿ���ԣ���Ϊ��
��1���ڸɾ��Թ��м���2ml2�� ��Ȼ�� �õ�������Һ����װ��5֧�Թܣ����Ϊ1#��2#��3#��4#��5# ��
��2�����εμ�2%��5%��10%��20%��40%����ȩ��Һ4�Σ�����������60��~70���ˮԡ�С�3���Ӻ��Թ�1#δ�γɴ�����������Թ�5# �������������кڰߣ� �Թ�4# ��������������һ�㣬�Թ�2#��3# �γɹ�����������
���о���Ŀ���ǣ� ��
��3����ȩ����������Ӧ�Ļ�ѧ����ʽΪ�� ��
��4����������:��ǿ���������£�����������Һ����������������֤�ͶԱ�ʵ�����¡�
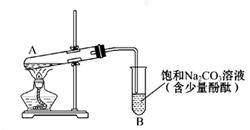
| װ�� | ʵ����� | �Թ��е�ҩƷ | ���� |
 | ʵ��� | 2mL������Һ�����ν�ŨNaOH��Һ | �����ݲ���: һ��ʱ�����Һ���:�Թܱڸ������� |
| ʵ��� | 2mL������Һ�� ����Ũ��ˮ | �����ݲ���:һ��ʱ�����Һ�����Ա仯 |
��ͬѧ������ʵ����ʵ���IJ��죬�������Ϻ�֪:
a��Ag(NH3)2++2H2O
 Ag++2NH3��H2O�� b��AgOH���ȶ������ֽ�Ϊ��ɫAg2O
Ag++2NH3��H2O�� b��AgOH���ȶ������ֽ�Ϊ��ɫAg2O�����飬ʵ������������NH3,��ɫ��������Ag2O������Ag2O��ԭ���ǣ�
�������������������� ��
��5����ʪ��ĺ�ɫʯ����ֽ����NH3�������������������������������������������� ����ϡHNO3�� ϴ�Թܱ��ϵ�Ag���÷�Ӧ�Ļ�ѧ����ʽ������������������ �� �������� ��
��6����ͬѧ�Բ���������ԭ���������:��NaOH�����£�������NH3��ԭAg2O������ü����������˵������ȩ��ʱ��������Һ���ܳ�ǿ���ԣ���Ϊ��
��1��AgNO3��Һ, ��μ���ϡ��ˮ,ֱ����������İ�ɫ������ʧΪֹ��
��2��̽��������Ӧʵ���У���ȩ��Һ��Ũ��������Ĺ�ϵ
��3��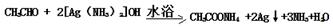
��4����NaOH�����£�����ʹNH3�ݳ�����ʹƽ��Ag(NH3)2 + + 2H2O ��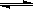 ��Ag++ 2NH3��H2O�����ƶ���c��Ag+������Ag+��OH����Ӧ����ת��ΪAg2O��
��Ag++ 2NH3��H2O�����ƶ���c��Ag+������Ag+��OH����Ӧ����ת��ΪAg2O��
��5����ֽ����; 4HNO3��ϡ��+ 3Ag��3AgNO3+ NO��+2H2O
��6����ǿ���������£�������Һ����Ҳ���γ����������뺬ȩ��������
��2��̽��������Ӧʵ���У���ȩ��Һ��Ũ��������Ĺ�ϵ
��3��
��4����NaOH�����£�����ʹNH3�ݳ�����ʹƽ��Ag(NH3)2 + + 2H2O ��
 ��Ag++ 2NH3��H2O�����ƶ���c��Ag+������Ag+��OH����Ӧ����ת��ΪAg2O��
��Ag++ 2NH3��H2O�����ƶ���c��Ag+������Ag+��OH����Ӧ����ת��ΪAg2O����5����ֽ����; 4HNO3��ϡ��+ 3Ag��3AgNO3+ NO��+2H2O
��6����ǿ���������£�������Һ����Ҳ���γ����������뺬ȩ��������
�����������2���ı���ȩŨ�ȣ��۲��������ԣ�̽��������Ӧʵ���У���ȩ��Һ��Ũ��������Ĺ�ϵ��
��4��Fe2��������������Fe3����Fe3����KSCN��Ӧ����Ѫ��ɫ��Fe2�����ܡ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
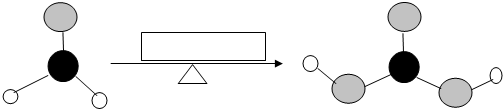

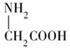 ���ͱ����ᣨ
���ͱ����ᣨ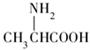 �����������γ�4�ֶ���
�����������γ�4�ֶ���