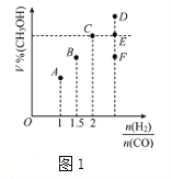
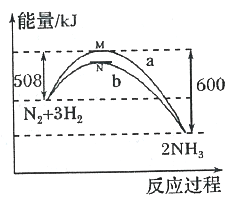
ЬтФПФкШн
ЁОЬтФПЁПЃЈ18ЗжЃЉДгБЁКЩгЭжаЕУЕНвЛжжЬўAЃЈC10H16ЃЉЃЌНаЈЛЁЊЗЧРМЬўЃЌгыAЯрЙиЗДгІШчЯТЃК

ЃЈ1ЃЉHЕФЗжзгЪНЮЊ ЁЃ
ЃЈ2ЃЉBЫљКЌЙйФмЭХЕФУћГЦЮЊ ЁЃ
ЃЈ3ЃЉКЌСНИіЁЊCOOCH3ЛљЭХЕФCЕФЭЌЗжвьЙЙЬхЙВга жжЃЈВЛПМТЧЪжадвьЙЙЃЉЃЌЦфжаКЫДХЙВеёЧтЦзГЪЯж2ИіЮќЪеЗхЕФвьЙЙЬхНсЙЙМђЪНЮЊ ЁЃ
ЃЈ4ЃЉBЁњDЃЌDЁњEЕФЗДгІРраЭЗжБ№ЮЊ ЁЂ ЁЃ
ЃЈ5ЃЉGЮЊКЌСљдЊЛЗЕФЛЏКЯЮяЃЌаДГіЦфНсЙЙМђЪНЃК ЁЃ
ЃЈ6ЃЉFдквЛЖЈЬѕМўЯТЗЂЩњОлКЯЗДгІПЩЕУЕНвЛжжИпМЖЮќЫЎадЪїжЌЃЌИУЪїжЌУћГЦЮЊ ЁЃ
ЃЈ7ЃЉаДГіEЁњFЕФЛЏбЇЗНГЬЪНЃК ЁЃ
ЃЈ8ЃЉAЕФНсЙЙМђЪНЮЊ ЃЌAгыЕШЮяжЪЕФСПЕФBr2НјааМгГЩЗДгІЕФВњЮяЙВга жжЃЈВЛПМТЧСЂЬхвьЙЙЃЉЁЃ
ЁОД№АИЁПЃЈ1ЃЉC10H20
ЃЈ2ЃЉєЪЛљ єШЛљ
ЃЈ3ЃЉ4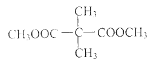
ЃЈ4ЃЉМгГЩЗДгІЃЈЛђЛЙдЗДгІЃЉ ШЁДњЗДгІ
ЃЈ5ЃЉ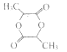
ЃЈ6ЃЉОлБћЯЉЫсФЦ
ЃЈ7ЃЉ
ЃЈ8ЃЉ![]() ЃЛ3
ЃЛ3
ЁОНтЮіЁП
ЪдЬтЃЈ1ЃЉИљОнHЕФНсЙЙМђЪНПЩЕУЗжзгЪНЮЊC10H20
ЃЈ2ЃЉBЕФНсЙЙМђЪНЮЊCH3![]() COOHЃЌЫљвдBЫљКЌЙйФмЭХЕФУћГЦЮЊєЪЛљ єШЛљ
COOHЃЌЫљвдBЫљКЌЙйФмЭХЕФУћГЦЮЊєЪЛљ єШЛљ
ЃЈ3ЃЉКЌСНИіЁЊCOOCH3ЛљЭХЕФCЕФЭЌЗжвьЙЙ ЁЂCH3OOCCH2CH2CH2COOCH3ЁЂCH3OOCCH2CH(CH3)COOCH3ЁЂCH3CH2C(COOCH3)2ЙВ4жжЃЛКЫДХЙВеёЧтЦзГЪЯж2ИіЮќЪеЗхЃЌМШHдзгЕФЮЛжУга2жжЃЌНсЙЙМђЪНЮЊЃК
ЁЂCH3OOCCH2CH2CH2COOCH3ЁЂCH3OOCCH2CH(CH3)COOCH3ЁЂCH3CH2C(COOCH3)2ЙВ4жжЃЛКЫДХЙВеёЧтЦзГЪЯж2ИіЮќЪеЗхЃЌМШHдзгЕФЮЛжУга2жжЃЌНсЙЙМђЪНЮЊЃК
ЃЈ4ЃЉBЁњDЮЊєЪЛљгыH2ЗЂЩњЕФМгГЩЗДгІЃЌDЁњEЮЊDжаЕФЈЛ-HдзгБЛBrШЁДњЃЌЗДгІРраЭЮЊШЁДњЗДгІЁЃ
ЃЈ5ЃЉDЗжзгФкєШЛљКЭєЧЛљЗЂЩњѕЅЛЏЗДгІЩњГЩGЃЌдђGЕФНсЙЙМђЪНЮЊЃК
ЃЈ6ЃЉEЮЊБћЯЉЫсЃЌгыNaOHДМШмвКЗДгІЩњГЩБћЯЉЫсФЦЃЌМгОлЗДгІПЩЕУFЃЌУћГЦЮЊЃКОлБћЫсФЦЁЃ
ЃЈ7ЃЉEдкNaOHДМШмвКЗЂЩњЯћШЅЗДгІКЭжаКЭЗДгІЃЌЫљвдEЁњFЕФЛЏбЇЗНГЬЪНЮЊЃК
ЃЈ8ЃЉИљОнBЁЂCЕФНсЙЙМђЪНКЭAЕФЗжзгЪН C10H16ПЩЭЦГіAЕФНсЙЙМђЪНЮЊЃК![]() ЃЛAжаСНИіЬМЬМЫЋМќгыЕШЮяжЪЕФСПЕФBr2ПЩЗжБ№НјааНјааМгГЩЗДгІЃЌвВПЩвдЗЂЩњ1,4МгГЩЃЌЫљвдВњЮяЙВга3жжЁЃ
ЃЛAжаСНИіЬМЬМЫЋМќгыЕШЮяжЪЕФСПЕФBr2ПЩЗжБ№НјааНјааМгГЩЗДгІЃЌвВПЩвдЗЂЩњ1,4МгГЩЃЌЫљвдВњЮяЙВга3жжЁЃ

 ЭЌВНСЗЯАЧПЛЏЭиеЙЯЕСаД№АИ
ЭЌВНСЗЯАЧПЛЏЭиеЙЯЕСаД№АИЁОЬтФПЁПКЌТШЛЏКЯЮядкЙЄХЉвЕЩњВњКЭШеГЃЩњЛюжагазХЙуЗКЕФгУЭОЁЃ
(1)ЙЄвЕЩЯгУТШЦјжЦБИЦЏАзЗлЃЌФГбаОПаЁзщРћгУЯТСазАжУжЦБИЦЏАзЗлЁЃ

ЂйЦЏАзЗлЕФгааЇГЩЗжЪЧ_____________ЃЈЬюЛЏбЇЪНЃЉЁЃЦЏАзЗлЗХжУдкПеЦјжавЛЖЮЪБМфКѓЛсЪЇаЇЃЌдвђЪЧЃЈаДГігаЙиЗДгІЕФЛЏбЇЗНГЬЪНЃЉ________________________ЁЃ
ЂкзАжУAжаЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЮЊ___________________________________ЁЃ
ЂлзАжУBжаЪдМСaЮЊ_________ЃЌзїгУЪЧ________________________________ЁЃ
(2)ClO2ГЃгУгкздРДЫЎЕФЯћЖОЁЂФОжЪжННЌЕФЦЏАзЁЃвбжЊЃКNaClЃЋ3H2O![]() NaClO3ЃЋ3H2ЁќЃЌ2NaClO3ЃЋ4HCl=2ClO2ЁќЃЋCl2ЁќЃЋ2NaClЃЋ2H2OЁЃгаЙиЮяжЪЕФШлЁЂЗаЕуШчЯТБэЃК
NaClO3ЃЋ3H2ЁќЃЌ2NaClO3ЃЋ4HCl=2ClO2ЁќЃЋCl2ЁќЃЋ2NaClЃЋ2H2OЁЃгаЙиЮяжЪЕФШлЁЂЗаЕуШчЯТБэЃК
ЮяжЪ | ШлЕу/Ёц | ЗаЕу/Ёц |
ClO2 | Ѓ59 | 11 |
Cl2 | Ѓ107 | Ѓ34.6 |
ClO2ЕФЩњВњСїГЬЪОвтЭМШчЯТЃК

РэТлЩЯУПЩњГЩ1molClO2ЃЌЭтНчжСЩйВЙГфXЦјЬх________ molЁЃДгClO2ЗЂЩњЦїжаЗжРыГіClO2ПЩВЩгУЕФЗНЗЈЪЧ____________ЁЃ