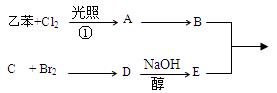
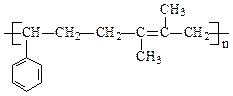��Ŀ����
��֪����ϩ����ˮ�ⷴӦ��R��CH��CH��O��R��  R��CH2CHO + R��OH������ϩ����A��ʽ��Ϊ176��������̼��ԭ����Ŀ��Ϊ3��4 ���л���B��ʽ��Ϊ60����صķ�Ӧ���¡���ش��������⣺
R��CH2CHO + R��OH������ϩ����A��ʽ��Ϊ176��������̼��ԭ����Ŀ��Ϊ3��4 ���л���B��ʽ��Ϊ60����صķ�Ӧ���¡���ش��������⣺
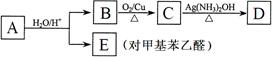
�� A�Ľṹ��ʽΪ________________________��B��������___________________��
��д��B �� C��Ӧ�Ļ�ѧ����ʽ��________________ _______________________��
_______________________��
��д�����ַ�������������E��ͬ���칹��Ľṹ��ʽ��__ ___________________��
___________________��
�� ���ڷ���ȩ�� �� �����������ֲ�ͬ����ԭ�ӡ�
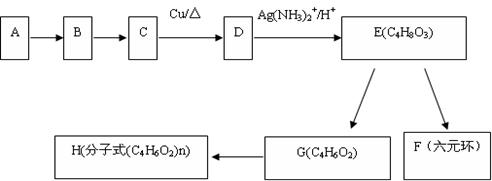
����Eת��Ϊ�Լ�����Ȳ��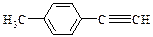 ����һ��·�����£�
����һ��·�����£�
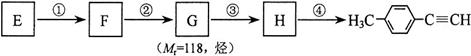
(I)д��G�Ľṹ��ʽ��_____________________________��
(II)����������Լ�����Ӧ����Ϊ��____________������ܵķ�Ӧ����Ϊ��________��
 R��CH2CHO + R��OH������ϩ����A��ʽ��Ϊ176��������̼��ԭ����Ŀ��Ϊ3��4 ���л���B��ʽ��Ϊ60����صķ�Ӧ���¡���ش��������⣺
R��CH2CHO + R��OH������ϩ����A��ʽ��Ϊ176��������̼��ԭ����Ŀ��Ϊ3��4 ���л���B��ʽ��Ϊ60����صķ�Ӧ���¡���ش��������⣺
�� A�Ľṹ��ʽΪ________________________��B��������___________________��
��д��B �� C��Ӧ�Ļ�ѧ����ʽ��________________
 _______________________��
_______________________����д�����ַ�������������E��ͬ���칹��Ľṹ��ʽ��__
 ___________________��
___________________���� ���ڷ���ȩ�� �� �����������ֲ�ͬ����ԭ�ӡ�
����Eת��Ϊ�Լ�����Ȳ��
 ����һ��·�����£�
����һ��·�����£�
(I)д��G�Ľṹ��ʽ��_____________________________��
(II)����������Լ�����Ӧ����Ϊ��____________������ܵķ�Ӧ����Ϊ��________��
��9�֣�
��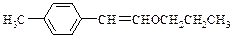 ��1��������������������2�֣�
��1��������������������2�֣�
�� 2CH3CH2CH2OH + O2 2CH3CH2CHO + H2O��2�֣�
2CH3CH2CHO + H2O��2�֣�
��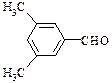
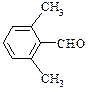
 �ȡ���2�֣�
�ȡ���2�֣�
�ȣ�I�� ��1�֣�
��1�֣�
��II��ŨH2SO4 ������ ��ȥ��Ӧ�� ��2�֣�
������ ��ȥ��Ӧ�� ��2�֣�
��
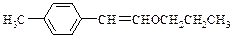 ��1��������������������2�֣�
��1��������������������2�֣��� 2CH3CH2CH2OH + O2
 2CH3CH2CHO + H2O��2�֣�
2CH3CH2CHO + H2O��2�֣���
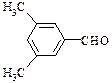
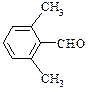
 �ȡ���2�֣�
�ȡ���2�֣��ȣ�I��
 ��1�֣�
��1�֣���II��ŨH2SO4
 ������ ��ȥ��Ӧ�� ��2�֣�
������ ��ȥ��Ӧ�� ��2�֣���

��ϰ��ϵ�д�
�����Ŀ
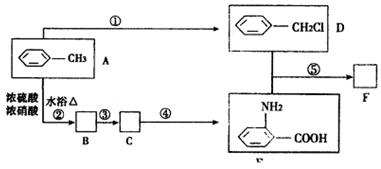
 RNHCH2 R/+HCl��R��R/����������
RNHCH2 R/+HCl��R��R/����������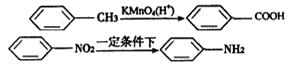
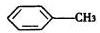 ��Ũ���ᡢŨ�������ڲ�ͬ�¶��»�õ���ͬ���
��Ũ���ᡢŨ�������ڲ�ͬ�¶��»�õ���ͬ���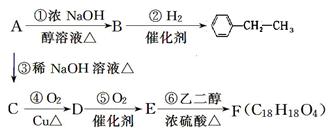
 ��36.5����ش�
��36.5����ش� �������������ԡ���������
�������������ԡ���������

 ____________
____________ _��II_________________��
_��II_________________�� CH2=CH2 + R1CH=CHR2
CH2=CH2 + R1CH=CHR2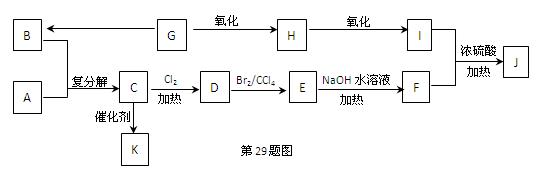
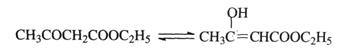
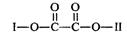 ������I��IIΪδ֪���ֵĽṹ����Ϊ�Ʋ�X�ķ��ӽṹ����������ͼ��ʾ��ת�����̣�
������I��IIΪδ֪���ֵĽṹ����Ϊ�Ʋ�X�ķ��ӽṹ����������ͼ��ʾ��ת�����̣�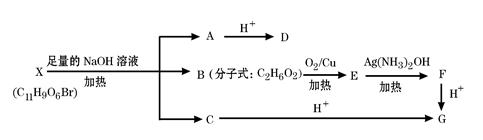
 һ�ֺ��������ŵ������ǣ� ��X�Ľṹ��ʽΪ�� ��
һ�ֺ��������ŵ������ǣ� ��X�Ľṹ��ʽΪ�� ��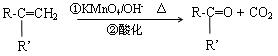 ��R��R����ʾ����������ţ�
��R��R����ʾ����������ţ�