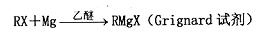��Ŀ����
��16�֣�
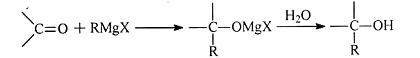
��֪��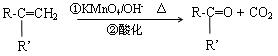 ��R��R����ʾ����������ţ�
��R��R����ʾ����������ţ�
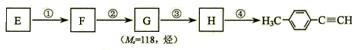
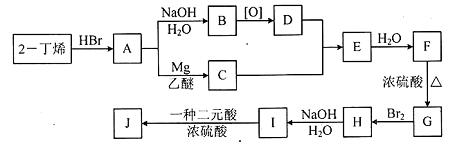
�л���A��һ��ҽҩ�м��壬����ͼ��ʾ����Է�������Ϊ130����֪0.5 mol A��ȫȼ��ֻ����3 mol CO2��2.5 mol H2O��A�ɷ�������ͼ��ʾ��ת��������D�ķ���ʽΪC4H6O2��������F��Ӧ��������Ԫ��״������

��ش�
��1��1 mol B�������Ľ��������ò���22.4 L����״����H2��B�����������ŵ������� ��
B��C����Է�������֮��Ϊ4��B��C�Ļ�ѧ����ʽ�� ��
��2��D��ͬ���칹��G������������D��ͬ����G�Ľṹ��ʽ������ �� ��
��3��F�ɷ����������͵ķ�Ӧ��
��������F��Ӧ���ɵ���Ԫ��״�������Ľṹ��ʽ�� ��
��F���Ƶ�ʹBr2��CCl4��Һ��ɫ���л���H��F��H�Ļ�ѧ����ʽ�� ��
��F��һ�������·������۷�Ӧ�Ļ�ѧ����ʽ�� ��
��4��A�Ľṹ��ʽ�� ��
��֪��
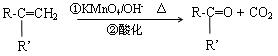 ��R��R����ʾ����������ţ�
��R��R����ʾ����������ţ��л���A��һ��ҽҩ�м��壬����ͼ��ʾ����Է�������Ϊ130����֪0.5 mol A��ȫȼ��ֻ����3 mol CO2��2.5 mol H2O��A�ɷ�������ͼ��ʾ��ת��������D�ķ���ʽΪC4H6O2��������F��Ӧ��������Ԫ��״������

��ش�
��1��1 mol B�������Ľ��������ò���22.4 L����״����H2��B�����������ŵ������� ��
B��C����Է�������֮��Ϊ4��B��C�Ļ�ѧ����ʽ�� ��
��2��D��ͬ���칹��G������������D��ͬ����G�Ľṹ��ʽ������ �� ��
��3��F�ɷ����������͵ķ�Ӧ��
��������F��Ӧ���ɵ���Ԫ��״�������Ľṹ��ʽ�� ��
��F���Ƶ�ʹBr2��CCl4��Һ��ɫ���л���H��F��H�Ļ�ѧ����ʽ�� ��
��F��һ�������·������۷�Ӧ�Ļ�ѧ����ʽ�� ��
��4��A�Ľṹ��ʽ�� ��
��1���ǻ���HOCH2-CH2OH + O2 OHC-CHO+ 2 H2O
OHC-CHO+ 2 H2O
 OHC-CHO+ 2 H2O
OHC-CHO+ 2 H2O
��

��ϰ��ϵ�д�
 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
�����Ŀ
 rdR.Schrock��ͬ��á��Ա�����
rdR.Schrock��ͬ��á��Ա����� ����ϩ�����ֽⷴӦ�����о���Ӧ���������Ĺ��ס���֪ϩ���Ľ��渴�ֽⷴӦ����Ϊ˫�����ѣ���λ���ӡ��ɱ�ʾΪ��
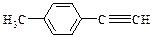
����ϩ�����ֽⷴӦ�����о���Ӧ���������Ĺ��ס���֪ϩ���Ľ��渴�ֽⷴӦ����Ϊ˫�����ѣ���λ���ӡ��ɱ�ʾΪ��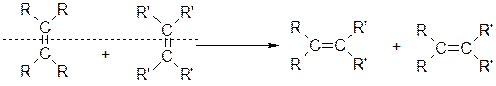
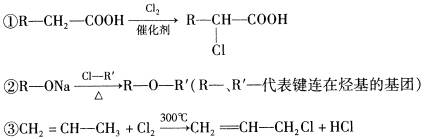
 R-CHCl-CH=CH2+HCl��
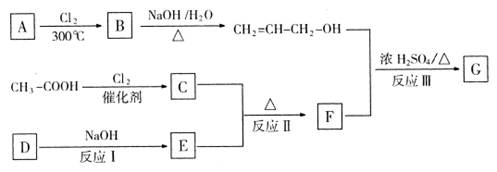
R-CHCl-CH=CH2+HCl�� �ȣ�FҲ������֬ˮ��õ����л���R�ĺϳ�·�����£�
�ȣ�FҲ������֬ˮ��õ����л���R�ĺϳ�·�����£�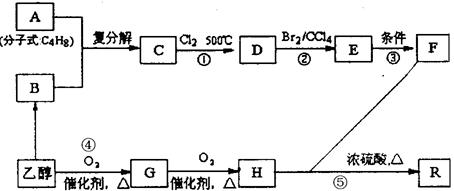
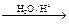 R��CH2CHO + R��OH������ϩ����A��ʽ��Ϊ176��������̼��ԭ����Ŀ��Ϊ3��4 ���л���B��ʽ��Ϊ60����صķ�Ӧ���¡���ش��������⣺
R��CH2CHO + R��OH������ϩ����A��ʽ��Ϊ176��������̼��ԭ����Ŀ��Ϊ3��4 ���л���B��ʽ��Ϊ60����صķ�Ӧ���¡���ش��������⣺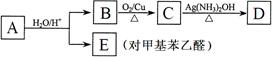
 _______________________��
_______________________�� ___________________��
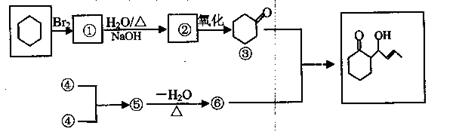
___________________�� ����һ��·�����£�
����һ��·�����£�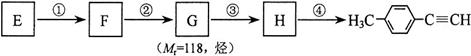
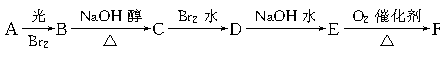
 ����ʹ��ˮ������ѧ��Ӧ����ɫ
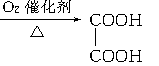
����ʹ��ˮ������ѧ��Ӧ����ɫ ģ������ͼ��ʾ��ͼ��������֮������߱�ʾ������˫������
ģ������ͼ��ʾ��ͼ��������֮������߱�ʾ������˫������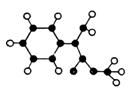

 E��F�ķ�Ӧ������ ��
E��F�ķ�Ӧ������ �� ��ʾ������X��Y����ΪH������д����������ͨʽ���ܷ���������Ӧ�������������ʵĽṹ��ʽ��
��ʾ������X��Y����ΪH������д����������ͨʽ���ܷ���������Ӧ�������������ʵĽṹ��ʽ��  R��CH2CHO + R��OH
R��CH2CHO + R��OH 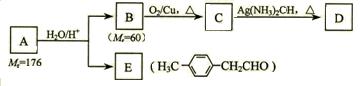
 ����һ��·�����£�
����һ��·�����£�