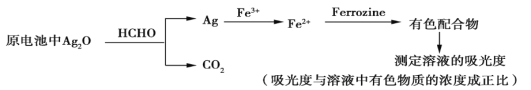
��Ŀ����
����Ŀ���һ������鳣��������ɫ�Ա���Ʒ��Ҳ�����л��ϳɡ�
(1)�һ�������(C8H16)�����Ʊ���Ȳ(C8H6)���Ȼ�ѧ����ʽ���£�
��C8H16(l)![]() C8H10(l)+3H2(g)����H1��0
C8H10(l)+3H2(g)����H1��0
��C8H10(l)![]() C8H6(l)+2H2(g)����H2��a kJ��mol-1
C8H6(l)+2H2(g)����H2��a kJ��mol-1
��C8H6(l)+5H2(g)![]() C8H16(l)����H3��b kJ��mol-1
C8H16(l)����H3��b kJ��mol-1
��Ӧ�ٵ���H1Ϊ__________(�ú�a��b�Ĵ���ʽ��ʾ)�����������������Ӧ�ٵ�ƽ��ת���ʵ�������____(����ĸ)��
A�����¸�ѹ��������B�����µ�ѹ��������C�����µ�ѹ��������D�����¸�ѹ
(2)��ͬѹǿ���¶����һ��������ƽ��ת��������ͼ��ʾ��

������ͬѹǿ�������¶ȣ�δ�ﵽ��ƽ��ǰ��v��____(������������С��������������)v����
���о��������������¶�������ѹǿ��C8H16(l)��ת����Ҳ���ߣ����ɿ�����____��
(3)t ����������ܱշ�Ӧ���г���1��00 mol C8H16(l)���д����⣬���Һ̬C8H10(l)��C8H6(l)�IJ���x1��x2(�����ʵ���������)��ʱ��仯��ϵ����ͼ��ʾ��

����8 hʱ����Ӧ��ϵ����������Ϊ_____mol(������������Ӧ)��Һ̬C8H16(l)��ת������_________��
��x1��������x2��ԭ����_____________��
���𰸡�(��b��a)kJ��mol��1 C ���� �����¶�ʱƽ�������ƶ��ij̶ȴ��ڼ�ѹʱƽ�������ƶ��ij̶�(������������) 1��951 40��1�� C8H16(l)ת��ΪC8H6(l)�Ļ��С����Ӧ���ʺܿ죬���ɵ�C8H10(l)��ת��ΪC8H6(l)�����x1��������x2
��������
��1�����ݸ�˹���ɿ�֪����=-����+�ۣ�������H1=-��a+b��kJ��mol��1���÷�Ӧ��S>0����H>0���ʿɲ��ø��µ�ѹʹƽ������̶��������ת���ʣ�
��2������ͼ������֪�������¶ȣ��һ��������ת�������������¶ȣ�ƽ�������ƶ����������¶ȣ��ﵽ��ƽ��ǰ��v������v����
�������¶ȣ�ƽ�������ƶ�������ѹǿ��ƽ�������ƶ����������¶ȵ��������ƶ��̶ȴ�������ѹǿ���������ƶ��̶�ʱ��C8H16(l)��ת���ʻ����ߣ�
��3���ٲμӷ�Ӧ��C8H16(l)Ϊ1mol��8hʱ��C8H6(l)�IJ���Ϊ0.374�������ʵ���Ϊ0.374mol��C8H10(l)�IJ���Ϊ0.027�������ʵ���Ϊ0.027mol����Ӧ���ɵ���������Ϊn��H2��=3����0.027mol+0.374mol��+2��0.374mol=1.951mol��n��C8H10(l)��+n��C8H6(l)��=0.401mol����Һ̬C8H16(l)��ת������![]() ��
��
��x1��������x2��ԭ����C8H16(l)ת��ΪC8H6(l)�Ļ��С����Ӧ���ʺܿ죬���ɵ�C8H10(l)��ת��ΪC8H6(l)�����x1��������x2��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ�������ģ��������ʽṹ�й����ʺ��ص㣬�ش��������⣺
��1������Ԫ��Co ��̬ԭ�Ӽ۵��ӹ������ʽΪ_______________________________�����ĵ�����I4 (Co) < I4 (Fe) ����ԭ����__________________________________________��
��2�����Ȼ��������֣�PCl3��PCl5 ��PCl3����ԭ�ӵ��ӻ�����Ϊ__________��PCl3�����幹��Ϊ__________������PCl3�ķе�________������ڡ���С�ڡ���PCl5��ԭ����__________��
��3��̪ݼ�����л�����̪ݼ��������γɵĸ��ӷ��ӣ��ṹ��ʽ����ͼ��ʾ���÷����д��ڵĻ�ѧ��Ϊ___________����ѡ����ĸ��
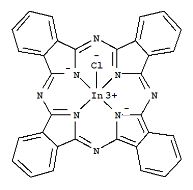
a���Ҽ� b���м� c�����Ӽ� d����λ��
��4�����ж��ֺ����ᣬ�����ƽ�ⳣ�����£�
��ѧʽ | HClO4 | HClO3 | HClO2 | HClO |
Ka | 1��1010 | 1��10 | 1��102 | 4��108 |
�����ʽṹ�ĽǶȽ������Ϻ����� Ka ���μ�С��ԭ��________________________��
��5���ܵ�һ�ֻ�����ľ����ṹ����ͼ��ʾ��
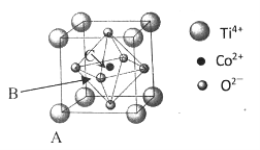
����֪A���ԭ���������Ϊ��0��0��0����C ��Ϊ��1/2��1/2��1/2������B���ԭ���������Ϊ___________��
����֪�������� a = 0.5485 nm����þ�����ܶ�Ϊ_______________g/cm3�����г��������ʽ���ɣ�