��Ŀ����
����Ŀ����I��ij�������к���C��N��H��O����Ԫ�أ���֪����ԭ���⣬����ԭ�Ӿ��ﵽ�����8���ӵ��ȶ��ṹ����ͼΪ�ð�������ӵ����ģ�ͣ�

(1)��������__________(����ۡ�������ά�ء����������ʡ�����֬��)��ȫˮ��IJ���ð�����Ľṹ��ʽΪ______________��
(2)�ð������к��������ŵ�������__________________��
(3)һ�������£��ð����������Ҵ�������Ӧ���˷�Ӧ�������������Ҵ��ķ�Ӧ��д���˷�Ӧ�Ļ�ѧ����ʽ��_______________________________________________________��
(4)��Ϊͬϵ����л��������ͬ�Ĺ����ţ���ð����ụΪͬϵ������һ��̼ԭ�ӵİ�����Ľṹ��ʽΪ_____________________��
��II����ͼ�������Թ����ȼ���2 mL 95%���Ҵ�������ҡ���»�������3 mLŨ���ᣬ�ټ���2 mL���ᣬ���ҡ�ȡ���ͼ���Ӻ�װ�ã��þƾ��ƶ����Թ�С�����3��5 min���ô����ȣ����۲쵽���Թ�������������ʱֹͣʵ�顣

�Իش�
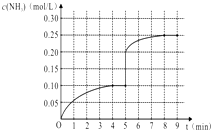
�������Թ���ͨ������________��Һ��ʵ�����ɵ��������������ܶȱ�ˮ________������������ζ��Һ�塣�������Թ����������������IJ���Ϊ(ֻ������)________��
�ڷ�Ӧ�м���Ũ�����������____________��_______________��
�۷�Ӧ�����ǣ�______________________ ��
���𰸡� ������  �Ȼ�
�Ȼ�  +CH3CH2OH
+CH3CH2OH![]()
![]() +H2O H2NCH2COOH ����̼���� С ��Һ ��ˮ�� ���� ȡ����Ӧ����������Ӧ��
+H2O H2NCH2COOH ����̼���� С ��Һ ��ˮ�� ���� ȡ����Ӧ����������Ӧ��
��������(I)�������ǵ�����ˮ��IJ����ͼ��֪�����ɫ��Ϊ̼ԭ�ӣ�С����Ϊ��ԭ�ӣ�����Ϊ��ԭ�ӣ��ݴ�д���ṹ��ʽ�������չ����ŵ����ʷ��������
(II)�����������������ñ���̼������Һ����������������ˮ��Һ������Ũ������������Ӧ�Ĵ�������ˮ����װ���з����ķ�Ӧ�Ǵ�����Ҵ���Ũ����������·���������Ӧ����������������ˮ��
(I)(1)��ͼ��֪���ṹ��ʽΪ �������ʽΪC3H7O3N���ʴ�Ϊ�������ʣ�
�������ʽΪC3H7O3N���ʴ�Ϊ�������ʣ� ��
��
(2) �к��������Ȼ������к������������Ȼ����ʴ�Ϊ���Ȼ���
�к��������Ȼ������к������������Ȼ����ʴ�Ϊ���Ȼ���
(3) ��-COOH�������Ҵ�����������Ӧ����ӦΪ
��-COOH�������Ҵ�����������Ӧ����ӦΪ +CH3CH2OH
+CH3CH2OH![]()
![]() +H2O���ʴ�Ϊ��
+H2O���ʴ�Ϊ�� +CH3CH2OH
+CH3CH2OH![]()
![]() +H2O��
+H2O��
(4)��Ϊͬϵ����л��������ͬ�Ĺ����ţ��� ��Ϊͬϵ������һ��̼ԭ�ӵİ�����ΪH2NCH2COOH���ʴ�Ϊ��H2NCH2COOH��
��Ϊͬϵ������һ��̼ԭ�ӵİ�����ΪH2NCH2COOH���ʴ�Ϊ��H2NCH2COOH��
(II)���������������ñ���̼������Һ��ԭ���У���������������ˮ�е��ܽ�ȣ�����̼���ƿ������ջӷ��������Ҵ������ᣬ�������Թ���ͨ�����뱥��̼������Һ��ʵ����ȡ�����������ḡ���ϲ㣬˵�������������ܶ�С��ˮ���ܶȣ���������������ˮ��Һ�����Է���ķ���Ϊ��Һ���ʴ�Ϊ������̼���ƣ�С����Һ��
��Ũ��������ˮ�ԣ��ٽ��÷�Ӧ������Ӧ�����ƶ���Ũ�����ܼӿ췴Ӧ���ʣ�����Ũ�������������ʴ�Ϊ����ˮ����������
��װ���з����ķ�Ӧ�Ǵ�����Ҵ���Ũ�������������������������ˮ����Ӧ�Ļ�ѧ����ʽΪ��CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O���÷�Ӧ����ȡ����Ӧ��������Ӧ���ʴ�Ϊ��ȡ����Ӧ(��������Ӧ)��
CH3COOCH2CH3+H2O���÷�Ӧ����ȡ����Ӧ��������Ӧ���ʴ�Ϊ��ȡ����Ӧ(��������Ӧ)��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��̼������(��ɫ���壬������ˮ)������Ҫ�Ĺ�ҵԭ�ϣ��������Ʊ���Ѫ������������ij�о�С�� ͨ������ʵ�飬Ѱ�����ø��ֽⷴӦ�Ƹ�FeCO3����ѷ���:
ʵ�� | �Լ� | ���� | |
�ι� | �Թ� | ||
| 0.8mol/LFeSO4��Һ(pH=4.5) | 1mol/LNa2CO3��Һ(pH=11.9) | ʵ����:������������ɫ��������������Եĺ��ɫ |
0.8mol/LFeSO4��Һ(pH=4.5) | 1mol/LNaHCO3��Һ(pH=8.6) | ʵ����: ������ɫ������������ɫ���ݣ�2min,��������ԵĻ���ɫ | |
0.8mol/L(NH4)2Fe(SO4)2��Һ(pH=4.0) | 1mol/LNaHCO3��Һ(pH=8.6) | ʵ����: ������ɫ��������ɫ���ݣ��ϳ�ʱ�䱣�ְ�ɫ | |
��1��ʵ��I�в���HCO3-�ͺ��ɫ���������ӷ���ʽΪ___________
��2��ʵ�����в���FeCO3�����ӷ���ʽΪ___________
��3��Ϊ��̽��ʵ������NH4+��������ã���ͬѧ�����ʵ��������̽��:
���� | ���� | |
ʵ���� | ��0.8mol/LFeSO4��Һ�м���_____���ټ���һ����Na2SO4�������Ƴɻ����Һ(��֪Na+��ʵ����Ӱ�죬���Ի�Ϻ���Һ����仯)����ȡ����Һһ�ιܣ���2mL1mol/LNaHCO3��Һ��� | ��ʵ����������ͬ |
ʵ�����м���Na2SO4�����Ŀ����_____
�Ա�ʵ��������ͬѧ�ó�����:NH4+ˮ�����H+,������ҺpH,�����˸�����Fe(OH)2�IJ�����
��ͬѧ��Ϊ��ʵ�鷽�������Ͻ���Ӧ����ĶԱ�ʵ�������_____.��ȡ����Һһ�ι�2mL 1mol/L NaHCO3��Һ��ϡ�
��4��С��ͬѧ��һ��������Ϊ,����ʵ��������ֱ��֤��ʵ������FeCO3�Ĵ�����ߡ���Ҫ������ͼ��ʾ��װ�ý��ж����ⶨ��

�ֱ�ʵ�����еij������й��ˡ�ϴ�ӡ�����������Ȼ��ת����A���Ĺ��ƿ�С���Ӧ��ɺ�ͨ��N2��������_______��Ϊ�ⶨFeCO3�Ĵ��ȣ�����Ʒ�������⣬����ⶨ����������_________
��5��ʵ�鷴˼:���ⶨ��ʵ�����е�FeCO3���ȸ���ʵ������ʵ������ͨ������ʵ��������Ʊ�FeCO3ʵ��ɹ��Ĺؼ�������__________.