��Ŀ����
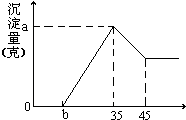 ȷ��ȡ6g������Al2O3��Fe2O3��SiO2����Ʒ������ʢ��100mL ϡH2SO4��Һ���ձ��У���ַ�Ӧ�����ȥ������������Һ�м���10mol/L��NaOH��Һ�����������������ͼ����NaOH��Һ�������mL����ͼ��ʾ���Իش��������⣺
ȷ��ȡ6g������Al2O3��Fe2O3��SiO2����Ʒ������ʢ��100mL ϡH2SO4��Һ���ձ��У���ַ�Ӧ�����ȥ������������Һ�м���10mol/L��NaOH��Һ�����������������ͼ����NaOH��Һ�������mL����ͼ��ʾ���Իش��������⣺
��1��������NaOH��Һ���������35mLʱ�������ķ�Ӧ�����ӷ���ʽΪ______
��2����������Al2O3�����ʵ����Ƕ��٣�
��3����b=2.6��ϡ������Һ�����ʵ���Ũ��Ϊ���٣�
�⣺��1����ͼ��֪������35mL����������Һʱ�����������NaOH��Һ���������35mLʱ����������������������������Ӧ��Al��OH��3+NaOH=NaAlO2+2H2O�����ӷ���ʽΪ��Al��OH��3+OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
��2����35mL��45mL�����10mL����������ȫ�ܽ������������ý����ĵ�n��NaOH��=0.01L��10mol/L=0.1mol�����ݷ���ʽAl��OH��3+NaOH=NaAlO2+2H2O��֪���������������ʵ���Ϊ0.1mol��������Ԫ���غ��֪n��Al2O3��=0.1mol�� =0.05mol��
=0.05mol��
����������Al2O3�����ʵ���Ϊ0.05mol��
��3��������NaOH��Һ�����Ϊ35mlʱ��n��NaOH��=0.035L��10mol/L=0.35mol��
��ʱ�����������ﵽ���ֵ�����ʱ��Һ������ֻ��Na2SO4��
����NaԪ���غ���n��Na2SO4��= n��NaOH��=
n��NaOH��= ��0.35mol=0.175mol��
��0.35mol=0.175mol��
����������غ㣬���У�n��H2SO4��=0.175mol��
����ԭ������Һ��c��H2SO4��=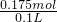 =1.75mol/L��
=1.75mol/L��
��ϡ������Һ�����ʵ���Ũ��Ϊ1.75mol/L��
��������1����ͼ��֪������35mL����������Һʱ�����������NaOH��Һ���������35mLʱ����������������������������Ӧ��Al��OH��3+NaOH=NaAlO2+2H2O��
��2����35mL��45mL�����10mL����������ȫ�ܽ���������������n=cV����ý����ĵ�n��NaOH�����ٸ��ݷ���ʽ�����������������ʵ�����������Ԫ���غ����n��Al2O3����
��3������35mL����������Һʱ�����������ʱ��Һ������Ϊ�����ƣ������������غ���������Ƶ����ʵ������ٸ���������غ����ԭ������Һ��n��H2SO4�����ٸ���c= ���㣮
���㣮
���������⿼��������йؼ��㣬�Ѷ��еȣ����ͼ���и��η����ķ�Ӧ�ǹؼ���ע������غ�ļ��㣬��3����ע�����÷�Ӧ�жϳ������ֵʱ����Һ�е����ʣ��������غ���㣬���Լ�����̣�
�ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
��2����35mL��45mL�����10mL����������ȫ�ܽ������������ý����ĵ�n��NaOH��=0.01L��10mol/L=0.1mol�����ݷ���ʽAl��OH��3+NaOH=NaAlO2+2H2O��֪���������������ʵ���Ϊ0.1mol��������Ԫ���غ��֪n��Al2O3��=0.1mol��
 =0.05mol��
=0.05mol������������Al2O3�����ʵ���Ϊ0.05mol��
��3��������NaOH��Һ�����Ϊ35mlʱ��n��NaOH��=0.035L��10mol/L=0.35mol��
��ʱ�����������ﵽ���ֵ�����ʱ��Һ������ֻ��Na2SO4��
����NaԪ���غ���n��Na2SO4��=
 n��NaOH��=
n��NaOH��= ��0.35mol=0.175mol��
��0.35mol=0.175mol������������غ㣬���У�n��H2SO4��=0.175mol��
����ԭ������Һ��c��H2SO4��=
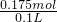 =1.75mol/L��
=1.75mol/L����ϡ������Һ�����ʵ���Ũ��Ϊ1.75mol/L��
��������1����ͼ��֪������35mL����������Һʱ�����������NaOH��Һ���������35mLʱ����������������������������Ӧ��Al��OH��3+NaOH=NaAlO2+2H2O��
��2����35mL��45mL�����10mL����������ȫ�ܽ���������������n=cV����ý����ĵ�n��NaOH�����ٸ��ݷ���ʽ�����������������ʵ�����������Ԫ���غ����n��Al2O3����
��3������35mL����������Һʱ�����������ʱ��Һ������Ϊ�����ƣ������������غ���������Ƶ����ʵ������ٸ���������غ����ԭ������Һ��n��H2SO4�����ٸ���c=
 ���㣮
���㣮���������⿼��������йؼ��㣬�Ѷ��еȣ����ͼ���и��η����ķ�Ӧ�ǹؼ���ע������غ�ļ��㣬��3����ע�����÷�Ӧ�жϳ������ֵʱ����Һ�е����ʣ��������غ���㣬���Լ�����̣�

��ϰ��ϵ�д�
 �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����Ŀ
 ȷ��ȡ6g������Al2O3��Fe2O3��SiO2����Ʒ������ʢ��100mL ϡH2SO4��Һ���ձ��У���ַ�Ӧ�����ȥ������������Һ�м���10mol/L��NaOH��Һ�����������������ͼ����NaOH��Һ�������mL����ͼ��ʾ���Իش��������⣺
ȷ��ȡ6g������Al2O3��Fe2O3��SiO2����Ʒ������ʢ��100mL ϡH2SO4��Һ���ձ��У���ַ�Ӧ�����ȥ������������Һ�м���10mol/L��NaOH��Һ�����������������ͼ����NaOH��Һ�������mL����ͼ��ʾ���Իش��������⣺ ��1�����к�Fe2O380%�ij�����100t�������Ͽ�ұ����Fe95%���������ٶ֣�
��1�����к�Fe2O380%�ij�����100t�������Ͽ�ұ����Fe95%���������ٶ֣�



