��Ŀ����
15��������Դ�����þ��й���ǰ������1�����в����ں�ˮ�����ķ���C������ţ���
A������ B�����ӽ����� C�����˷� D����������
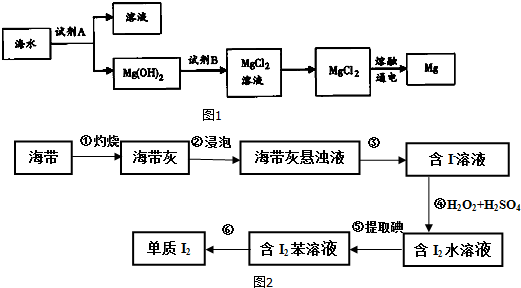
��2��ͼ1�ǴӺ�ˮ����ȡþ�ļ����̣�
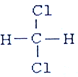
���Լ�B��HCl���ѧʽ����
������ˮMgCl2��ȡMg�Ļ�ѧ����ʽ��MgCl2�����ڣ�$\frac{\underline{\;���\;}}{\;}$Mg+Cl2����
��3���������и�����I-��ʽ���ڵĵ�Ԫ�أ�ʵ������ȡI2��;����ͼ2��ʾ��
I�����в����ڲ���ٲ������õ�������B������ţ���
A���ƾ��� B��©�� C������ D��������
����۵�ʵ����������ǹ��ˣ�
�ܲ��跴Ӧ�����ӷ���ʽH2O2+2H++2I-=I2+2H2O��
���������һ�ּ�����ȡ����ˮ��Һ���Ƿ��еⵥ�ʵļ���ȡ����ȡ�⣨�ݣ����ˮ��Һ���������Թ��У��μӵ��ۣ�����Һ�Ƿ�������������ˮ��Һ�л����еⵥ�ʣ�
���� ��1����ˮ����ˮ��Ӧ��ˮ���η��룬�����������������ӽ������ȣ�
��2������ȡMg�����̿�֪����ˮ�м��Լ�AΪ��ʯ�ң���þ����ת��Ϊ�������Լ�BΪ���ᣬ����������Ȼ�þ�õ�Mg��
��3����ʵ������ȡI2��;����֪�����������������գ��ܽ����˵õ���I-����Һ���ӹ������������õ����ⵥ�ʵ���Һ����Ϊ��ȡ����Ϊ����õ��⣬�Դ������
��� �⣺��1��A�������ǰ�ˮ��ˮ�Ļ�����з���������õ�������ˮ����A��ѡ��
B��ͨ�����ӽ�����֬���Գ�ȥ��ˮ�е����ӣ��Ӷ��ﵽ������ˮ��Ŀ�ģ���B��ѡ��
C������ֻ�ܳ�ȥˮ�еIJ��������ʣ����ܵ�����ˮ����Cѡ��
D�����õ���������ʹ��Ӧ������ͨ����Ĥ�Դﵽ������ˮ��Ч������D��ѡ��
�ʴ�Ϊ��C��
��2������ȡMg�����̿�֪����ˮ�м��Լ�AΪ��ʯ�ң���þ����ת��Ϊ�������Լ�BΪ���ᣬ����������Ȼ�þ�õ�Mg��
��������������֪��BΪHCl���ʴ�Ϊ��HCl��
������ˮMgCl2��ȡMg�Ļ�ѧ����ʽ��MgCl2�����ڣ�$\frac{\underline{\;���\;}}{\;}$Mg+Cl2�����ʴ�Ϊ��MgCl2�����ڣ�$\frac{\underline{\;���\;}}{\;}$Mg+Cl2����
��3����ʵ������ȡI2��;����֪�����������������գ��ܽ����˵õ���I-����Һ���ӹ������������õ����ⵥ�ʵ���Һ����Ϊ��ȡ����Ϊ����õ��⣬
I������ٲ�����Ҫ�ƾ��ơ������������ǣ�����Ҫ©�����ʴ�Ϊ��B��
II��������������֪������۵�ʵ����������ǹ��ˣ��ʴ�Ϊ�����ˣ�
�ܲ��跴Ӧ�����ӷ���ʽΪH2O2+2H++2I-=I2+2H2O���ʴ�Ϊ��H2O2+2H++2I-=I2+2H2O��
IV��������ȡ����ˮ��Һ���Ƿ��еⵥ�ʵļ���Ϊȡ����ȡ�⣨�ݣ����ˮ��Һ���������Թ��У��μӵ��ۣ�����Һ�Ƿ�������������ˮ��Һ�л����еⵥ�ʣ��ʴ�Ϊ��ȡ����ȡ�⣨�ݣ����ˮ��Һ���������Թ��У��μӵ��ۣ�����Һ�Ƿ�������������ˮ��Һ�л����еⵥ�ʣ�
���� ���⿼�麣ˮ��Դ���ۺ�Ӧ�ã�Ϊ��Ƶ���㣬�������̷��������������ᴿ���������ʵ�����Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬��Ŀ�Ѷ��еȣ�

 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�| A�� | ���ʷ�����ѧ��Ӧʱ�������������仯�����������仯�ı仯һ���ǻ�ѧ�仯 | |
| B�� | ��Ҫ���ȵĻ�ѧ��Ӧһ�������ȷ�Ӧ������Ҫ���Ⱦ��ܽ��еķ�Ӧһ���Ƿ��ȷ�Ӧ | |
| C�� | ���������������������ֱ���ȫȼ�գ�ǰ�߷ų��������� | |
| D�� | ��Ϊ3O2�T2O3�����ȷ�Ӧ�����Գ������������ȶ� |
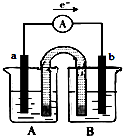
| A�� | a��Ϊͭ��b��Ϊп | |
| B�� | A����ʢ�ŵ���CuSO4��Һ��B����ʢ�ŵ���ZnSO4��Һ | |
| C�� | ��������������64gʱ������������С65g | |
| D�� | �����е���������п���ƶ�����������ͭ���ƶ� |
| A�� | �ñ���Na2CO3 ��Һ��ȥCO2�к��е�HCl | |
| B�� | ����ɫZnS�����еμ�CuSO4��Һ��������ڣ�֤����Ksp��CuS����Ksp��ZnS�� | |
| C�� | ��������к͵ζ�ʱ���۾�Ҫע�ӵι�Һ��ı仯 | |
| D�� | �к��Ȳⶨʵ��ʱ��Ӧ��NaOH��Һ�������������� |
| A�� |  �Ʊ��������������۲�����ɫ | B�� | 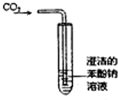 ̼�ᡢ��������ǿ���Ƚ� | ||
| C�� | 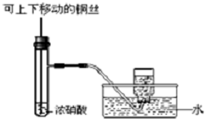 �Ʊ����ռ�����NO2���� | D�� | 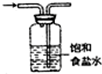 ��ȥ�������Ȼ��� |
| A�� | 2Na+2H2O�T2NaOH+H2�� | B�� | 4Fe��OH��2+O2+2H2O�T4Fe��OH��3 | ||
| C�� | 2F2+2H2O�T4HF+O2 | D�� | CaO+H2O�TCa��OH��2 |

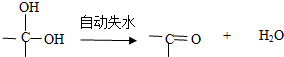
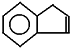 ������һ��ͬ���칹��A��A�����к���һ��������ֻ��һ��������A������ת����ϵ��
������һ��ͬ���칹��A��A�����к���һ��������ֻ��һ��������A������ת����ϵ��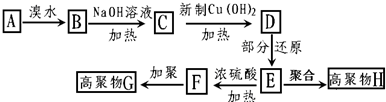
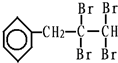 ��C�Ľṹ��ʽ��
��C�Ľṹ��ʽ��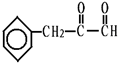
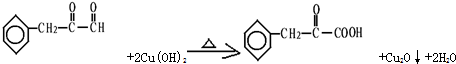

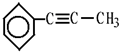
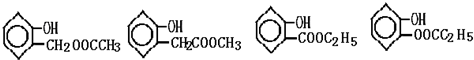 ����дһ����
����дһ����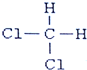 ��
��