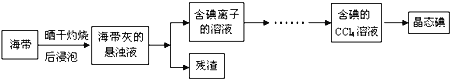
��Ŀ����
����Ŀ��þ����Ͻ���һ����;�ܹ�Ľ������ϣ�Ŀǰ������60%��þ�ǴӺ�ˮ����ȡ�ġ���Ҫ����:
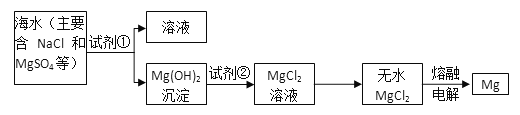
�Իش��������⣺
��1��Ϊ��ʹMgSO4ת��ΪMg(OH)2���Լ��ٿ���ѡ��__________________________��
��2�������Լ��ٺ��ܹ�����õ�Mg(OH)2�����ķ�����____________________��
��3���Լ��ڿ���ѡ��_______����Ӧ�����ӷ���ʽΪ��__________________________��
��4����ˮMgCl2������״̬�£�ͨ�������Mg��Cl2��д���÷�Ӧ�Ļ�ѧ����ʽ________________________��
���𰸡� ������ʯ��������NaOH ���� ���� Mg(OH)2��2H��===Mg2����2H2O MgCl2(����)![]() Mg+Cl2��
Mg+Cl2��
��������(1)MgSO4��NaOH��ʯ���鷴Ӧ������Mg(OH)2�����ӷ���ʽΪMg2++2OH-�TMg(OH)2����ҪʹMgSO4��ȫת��Ϊ�����������Լ�����Ӧ�������ʴ�Ϊ��������ʯ�����NaOH��
(2)��Mg(OH)2����������ˮ�����ù��˵ķ������룬�ʴ�Ϊ�����ˣ�
(3)Mg(OH)2��HCl��Ӧ������MgCl2��H2O������ʽΪ��Mg(OH)2+2HCl=MgCl2+2H2O���ʴ�Ϊ������(HCl)��Mg(OH)2+2H+=Mg2++2H2O��
(4)��ˮMgCl2������״̬�£�ͨ�������Mg��Cl2��MgCl2(����)![]() Mg+Cl2�����ʴ�Ϊ��MgCl2(����)
Mg+Cl2�����ʴ�Ϊ��MgCl2(����)![]() Mg+Cl2����
Mg+Cl2����

 ����ѧ��Ӧ�����ϵ�д�
����ѧ��Ӧ�����ϵ�д�