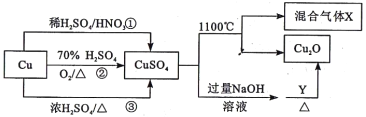
��Ŀ����
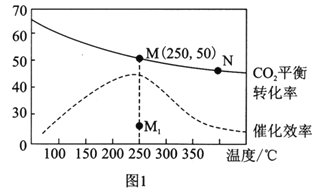
����Ŀ�����ݻ�Ϊ2.0 L���ܱ������ڣ�����D��T ��ʱ������Ӧ���䷴Ӧ�������������ʵ�����ʱ��t�ı仯��ϵ����ͼ����������������ǣ� ��

A. ������ͼ�÷�Ӧ��ƽ�ⳣ������ʽΪ![]()
B. ���ڵ�7����ʱ����D�����ʵ�����A�����ʵ����仯�������a����
C. ���ڵ�5����ʱ�����¶ȣ���÷�Ӧ������Ӧ�����ȷ�Ӧ����Ӧ��ƽ�ⳣ������B�ķ�Ӧ��������
D. �ӷ�Ӧ��ʼ����һ�δﵽƽ��ʱ��A���ʵ�ƽ����Ӧ����Ϊ0. 067mol/(L��min)
���𰸡�B
��������ƽ��ʱD�����ʵ���������0.4mol��A�����ʵ���������0.4mol��B�����ʵ���������0.2mol����D��A��B�����ʵ����仯��֮����2:2:1�����Ը÷�Ӧ�Ļ�ѧ����ʽΪ2D��s��![]() 2A��g����B��g�����÷�Ӧ��ƽ�ⳣ������ʽΪK��c��A��2��c��B����A����ȷ��D�ǹ��壬�ı�����������ƽ�ⲻ�ƶ������ʾA�����ʵ����仯��b���ߣ�B����������¶ȣ���ѧ��Ӧ���ʼӿ죬������Ӧ�����ȷ�Ӧ���������¶�ƽ��������Ӧ�����ƶ�����Ӧ��ƽ�ⳣ��������C����ȷ���ӷ�Ӧ��ʼ����һ�δﵽƽ��ʱ����һ�δﵽƽ��ʱ��A����������0.4mol����A��ƽ����Ӧ����Ϊ
2A��g����B��g�����÷�Ӧ��ƽ�ⳣ������ʽΪK��c��A��2��c��B����A����ȷ��D�ǹ��壬�ı�����������ƽ�ⲻ�ƶ������ʾA�����ʵ����仯��b���ߣ�B����������¶ȣ���ѧ��Ӧ���ʼӿ죬������Ӧ�����ȷ�Ӧ���������¶�ƽ��������Ӧ�����ƶ�����Ӧ��ƽ�ⳣ��������C����ȷ���ӷ�Ӧ��ʼ����һ�δﵽƽ��ʱ����һ�δﵽƽ��ʱ��A����������0.4mol����A��ƽ����Ӧ����Ϊ![]() 0.067mol/��L��min����D����ȷ��
0.067mol/��L��min����D����ȷ��

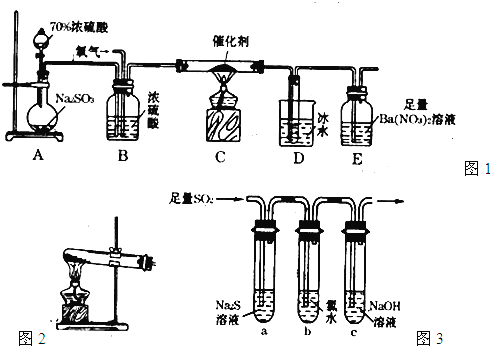
����Ŀ����������(POCl3)����Ҫ�Ļ�������ԭ�ϣ��㷺������ҩ��Ⱦ�����ܽ���������ҵ��ij��ȤС��ģ��PCl3ֱ���������Ʊ�POCl3,ʵ��װ���������:

�й����ʵIJ����������±�:
�۵�/�� | �е�/�� | ���� | |
PCl3 | -112 | 75.5 | ��ˮ����H3PO3��HCl����O2����POCl3 |
POCl3 | 2 | 105.3 | ��ˮ����H3PO4��HCl,������PCl3 |
�ش���������:
(1)����a��������_____��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ____________��
(2)Bװ�õ����ó��۲�O2������֮�⡣����____________��
(3)Cװ�ÿ��Ʒ�Ӧ��60�桫65����У�����ҪĿ����____________��
(4)ͨ��������·����Բⶨ�������ײ�Ʒ��ClԪ�غ�����ʵ�鲽������:
I.ȡxg��Ʒ����ƿ�У���������NaOH��Һ������ȫ��Ӧ���ϡ���������ԡ�
II.����ƿ�м���0.1000mol/L��AgNO3��Һ40.00mL,ʹCl-��ȫ������
III.�����м���2mL������������ҡ����ʹ�������汻�л��︲�ǡ�
IV.����ָʾ������cmol/LNH4SCN��Һ�ζ�����Ag+���յ㣬�����������VmL��
��֪:Ksp(AgCl)=3.2��10-10,Ksp(AgSCN)=2��10-12
�ٵζ�ѡ�õ�ָʾ����_______(����)���ζ��K�������Ϊ_____________��
a.FeCl2 b.NH4Fe(SO4)2 c.���� d.����
��C1Ԫ�ص������ٷֺ���Ϊ(�г���ʽ)____________��
�۲���III������������Ŀ����___�����˲���������C1Ԫ�غ�������___(�ƫ��ƫС�����䡱)��
����Ŀ������ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ��_________��(��"��"��"��")����Һ������_________ϴ��(������)����֪��
�ܶ�(g/cm3) | �۵�(��) | �е�(��) | �ܽ��� | |
������ | 0.96 | 25 | 161 | ������ˮ |
����ϩ | 0.81 | -103 | 83 | ������ˮ |
A��KMnO4��Һ B��ϡH2SO4 C�� Na2CO3��Һ