��Ŀ����
�������Ʊ��治�����ױ��ʣ�����Na2CO3��
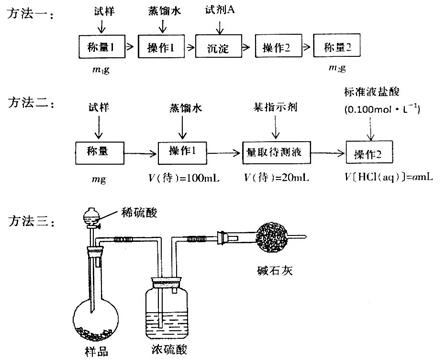
��1��ij����������Ʒ�Ѿ����ֱ��ʣ�����ѡ��һ����Һ ��֤�����������Ѿ����ʡ�
��2��ij����С��Ϊ�˴��Բⶨ�������Ƶ��������������dz�ȡag��Ʒ�����������ͼװ�����ⶨ�������Ƶ�����������
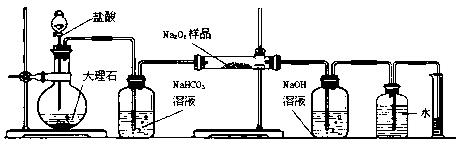
��ͼ�е�E��F��������װ�ã������ⶨO2�������
��д��װ��B�з�����Ӧ�����ӷ���ʽ��
��NaOH��������
�������ڶ�����Ͳ��ˮ�������������ɱ�״�������������ΪVmL������Ʒ�й������Ƶ���������Ϊ
��1��ij����������Ʒ�Ѿ����ֱ��ʣ�����ѡ��һ����Һ ��֤�����������Ѿ����ʡ�
��2��ij����С��Ϊ�˴��Բⶨ�������Ƶ��������������dz�ȡag��Ʒ�����������ͼװ�����ⶨ�������Ƶ�����������

| A�� | B�� | C�� | D����E����F�� |
��д��װ��B�з�����Ӧ�����ӷ���ʽ��
��NaOH��������
�������ڶ�����Ͳ��ˮ�������������ɱ�״�������������ΪVmL������Ʒ�й������Ƶ���������Ϊ
��1��BaCl2��Һ
��2����HCO3��+H+ = H2O+CO2�� ������δ��Ӧ��CO2 ��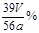
��2����HCO3��+H+ = H2O+CO2�� ������δ��Ӧ��CO2 ��
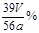
�����������1���������Ʊ��治�����ױ��ʣ�����Na2CO3���ʼ����������Ƿ���ʣ�ֻ�����CO32-����ѡ��BaCl2��Һ����2��װ��B��̼��������ӷ����������ᷴӦ�����ӷ�Ӧ����ʽΪ����HCO3��+H+ = H2O+CO2������NaOH������������δ��Ӧ��CO2����3������Ʒ�й������Ƶ����ʵ���Ϊ��n
2Na2O2 +2H2O�T4NaOH+O2��
2 1
n V��10-3L/22��4L/mol
��ã�n= 2V��10-3L/22��4L/mol����Na2O2������Ϊ78��2V��10-3L/22��4L/mol������Ʒ�й������Ƶ���������Ϊ[��78��2V��10-3g/22��4��/ag] ��100%=
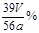

��ϰ��ϵ�д�
 �㽭��У��ʦ���ϵ�д�
�㽭��У��ʦ���ϵ�д�
�����Ŀ