��Ŀ����
���ֽ���������ˮ��һ�������·�����Ӧ��
��1����һС�������Ͷ��ʢ��ˮ���ձ��У�����ȫ��Ӧ�������еμӷ�̪��Һ��������ˮ��Ӧ�Ļ�ѧ����ʽ�� ��
������ʵ������У��ɹ۲쵽��ʵ�������� ����ѡ����ţ���
��������b��ԭ���� ��
a���Ƹ���ˮ�� b�����۳�С��
c��������ˮ�����Ĵ��ζ�������˻˻��������ʧ
d����Ӧ�����ձ��еμӷ�̪��Һ����Һ�ʺ�ɫ
��2�����ۿ�����ˮ�����ڸ����·�Ӧ������ ���ѧʽ����������
��1����һС�������Ͷ��ʢ��ˮ���ձ��У�����ȫ��Ӧ�������еμӷ�̪��Һ��������ˮ��Ӧ�Ļ�ѧ����ʽ�� ��
������ʵ������У��ɹ۲쵽��ʵ�������� ����ѡ����ţ���
��������b��ԭ���� ��
a���Ƹ���ˮ�� b�����۳�С��
c��������ˮ�����Ĵ��ζ�������˻˻��������ʧ
d����Ӧ�����ձ��еμӷ�̪��Һ����Һ�ʺ�ɫ
��2�����ۿ�����ˮ�����ڸ����·�Ӧ������ ���ѧʽ����������
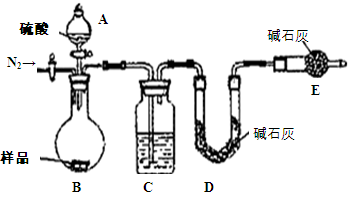
��1����2Na + 2H2O == 2NaOH + H2�� ��a b c d ��Ӧ�ų��������ȣ��Ƶ��۵�ϵͣ�2��Fe3O4
�����������1������ˮ��Ӧ�Ļ�ѧ����ʽ�Ǣ�2Na + 2H2O == 2NaOH + H2����������Na���ܶȱ�ˮС�����Ը���ˮ���ϣ�����Na���۵�ͣ�Na��ˮ�ķ�Ӧ���Ƿ��ȷ�Ӧ����Ӧ�ų�������Na�ۻ����������۳�С��Na��ˮ��Ӧ�������������������Ѳ���˻˻��������Na���ܵ��ĸ������������С���ȣ�������ˮ�����Ĵ��ζ��� Na��ˮ��Ӧ����NaOH��ʹ��Һ�Լ��ԣ��μӷ�̪�Լ�����Һ��Ϊ��ɫ����2�����ۿ�����ˮ�����ڸ����·�Ӧ������ʽΪ3Fe+4H2O(g)
 Fe3O4+4H2�����ɼ���Ӧ������Fe3O4��������
Fe3O4+4H2�����ɼ���Ӧ������Fe3O4��������
��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
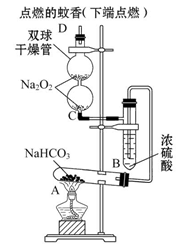
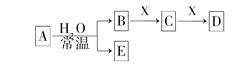
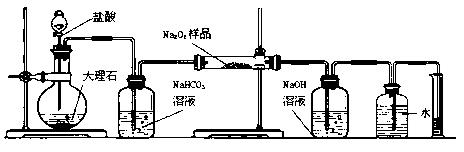
 �ⶨʣ���������
�ⶨʣ��������� �ⶨ��������
�ⶨ�������� �ⶨ���ɶ�����̼������
�ⶨ���ɶ�����̼������