��Ŀ����
CuSO4��Һ��Na2CO3��Һ��ϲ�������ɫ������������ij��ȤС��Գ�����ɵ�̽����
��������衿
����1������ΪCu (OH)2
����2������Ϊ
����3������Ϊ��ʽ̼��ͭ[��ѧʽ�ɱ�ʾΪnCuCO3��mCu (OH)2]
���������ϡ���������һ�ֳ������Ⱦ��ֽ⣨����������ᾧˮ����
������̽����
����1������������Һ���ˣ�������ˮϴ�ӣ�������ˮ�Ҵ�ϴ�ӣ���ɣ�
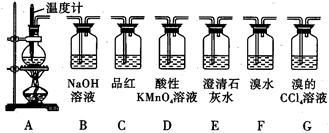
����2����ͬѧȡһ�������壬�����������õ�����װ�ã��г�����δ���������ж���ʵ�飻
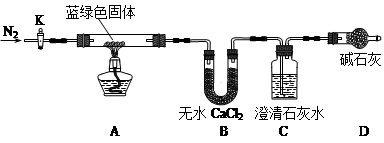
��1������Ӧ��A������ɫ�����ڣ�C������������֤������ ������
��2����ͬѧ��ΪֻҪ����ͼ��Bװ�õ��Լ���������ij�Լ������֤�������м��裬���Լ��� ������ţ���
a��Ũ���� b����ˮCuSO4 c����ʯ�� d��P2O5
��3����ͬѧ����B�Լ�����֤����3������ʵ�������� ��
������̽����
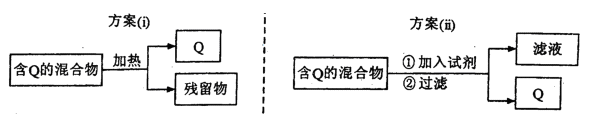
��4����ͬѧ��һ��̽������3�й������ɣ�
����ͬѧ���һЩ������20������ݣ����±�����C�еij���ʯ��ˮ��ΪBa(OH)2��Һ����ԭ���� ��˫ѡ������ţ�
a��Ba(OH)2�ܽ�ȴ���Ca(OH)2���ܳ������CO2
b��Ba(OH)2Ϊǿ�Ca(OH)2Ϊ����
c�����յ���CO2���ɵ�BaCO3����������CaCO3���������С
d����ͬ�����£�CaCO3���ܽ�����Դ���BaCO3
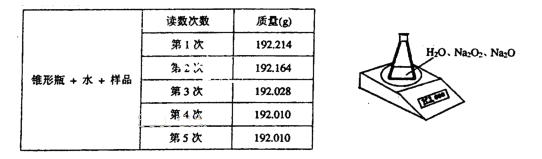
������ȡ����ɫ��������Ϊ54.2 g��ʵ�������װ��B����������5.4 g��C�еIJ�������������Ϊ39.4 g���������ɫ����Ļ�ѧʽΪ ��
��������衿
����1������ΪCu (OH)2
����2������Ϊ
����3������Ϊ��ʽ̼��ͭ[��ѧʽ�ɱ�ʾΪnCuCO3��mCu (OH)2]
���������ϡ���������һ�ֳ������Ⱦ��ֽ⣨����������ᾧˮ����
������̽����
����1������������Һ���ˣ�������ˮϴ�ӣ�������ˮ�Ҵ�ϴ�ӣ���ɣ�
����2����ͬѧȡһ�������壬�����������õ�����װ�ã��г�����δ���������ж���ʵ�飻

��1������Ӧ��A������ɫ�����ڣ�C������������֤������ ������
��2����ͬѧ��ΪֻҪ����ͼ��Bװ�õ��Լ���������ij�Լ������֤�������м��裬���Լ��� ������ţ���
a��Ũ���� b����ˮCuSO4 c����ʯ�� d��P2O5
��3����ͬѧ����B�Լ�����֤����3������ʵ�������� ��
������̽����
��4����ͬѧ��һ��̽������3�й������ɣ�
����ͬѧ���һЩ������20������ݣ����±�����C�еij���ʯ��ˮ��ΪBa(OH)2��Һ����ԭ���� ��˫ѡ������ţ�
| �ܽ��(S)/g | �ܶȻ�(Ksp) | Ħ������(M)/g��mol��1 | |||
| Ca(OH)2 | Ba(OH)2 | CaCO3 | BaCO3 | CaCO3 | BaCO3 |
| 0.16 | 3.89 | 2.9��10-9 | 2.6��10-9 | 100 | 197 |
b��Ba(OH)2Ϊǿ�Ca(OH)2Ϊ����
c�����յ���CO2���ɵ�BaCO3����������CaCO3���������С
d����ͬ�����£�CaCO3���ܽ�����Դ���BaCO3
������ȡ����ɫ��������Ϊ54.2 g��ʵ�������װ��B����������5.4 g��C�еIJ�������������Ϊ39.4 g���������ɫ����Ļ�ѧʽΪ ��
CuCO3��1�֣�
��1��1��1�֣�
��2��b��2�֣���
��3��A������ɫ������ɫ��B����ˮCuSO4���������C���а�ɫ����������3�֣���A��B��C��ÿ�������1�֡�
��4����ac��2�֣���©һ����1�֣���һ������1�֣�����Ϊֹ��
��2CuCO3��3Cu(OH)2����3Cu(OH)2��2CuCO3��Cu5(OH)6(CO3)2��3�֣�
��1��1��1�֣�
��2��b��2�֣���
��3��A������ɫ������ɫ��B����ˮCuSO4���������C���а�ɫ����������3�֣���A��B��C��ÿ�������1�֡�
��4����ac��2�֣���©һ����1�֣���һ������1�֣�����Ϊֹ��
��2CuCO3��3Cu(OH)2����3Cu(OH)2��2CuCO3��Cu5(OH)6(CO3)2��3�֣�
���������CuSO4��Һ��Na2CO3��Һ��ϲ�������ɫ������ԭ�������ͭ���Ӻ�̼������ӷ���˫ˮ�ⷴӦ����Cu (OH)2������ͭ������̼������ӽ������CuCO3�������������෴Ӧ������ʱ���ɵļ�ʽ̼��ͭ��������1����ʵ�������֪������ɫ�������ȷֽ���CO2���ɣ�˵�����������̼��ͭ����ֻ�м���1��������2����ɫ����ˮCuSO4��ˮ���������Bװ�õ���ˮ�Ȼ��Ƹ�Ϊ��ˮ����ͭ�������֤�������м��裻��A�й����Ϊ��ɫ��B�а�ɫ���������C�г���ʯ��ˮ����ǣ������3��������4��Ba(OH)2��Ca(OH)2�ܽ�ȷֱ�Ϊ3.89g��0.16g����a��ȷ��Ba(OH)2��Ca(OH)2����ǿ���b���� BaCO3��CaCO3Ħ����������ֵ�ֱ�Ϊ197��100����c��ȷ��CaCO3��BaCO3�ܶȻ�����С����d����5���������֪m(H2O)=5.4g��m(BaCO3)=39.4g����m/M��֪��n(H2O)=0.3mol��n(BaCO3)=0.2mol�����⡢̼�غ��ϵʽnCuCO3��mCu (OH)2��mH2O��nCO2��nBaCO3��֪��m/n=0.3/0.2=3/2��������ʻ�ѧʽΪ�ֱ�Ϊ2CuCO3��3Cu(OH)2����3Cu(OH)2��2CuCO3��Cu5(OH)6(CO3)2��

��ϰ��ϵ�д�
 ��������ϵ�д�
��������ϵ�д�
�����Ŀ