��Ŀ����
1�������Ԫ�����ڱ���һ���֣��������е���ĸ�ֱ����ijһ��ѧԪ�أ�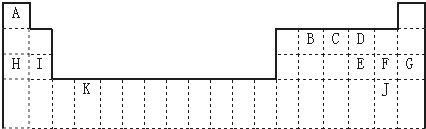
��1����������ĸ�����Ԫ���У���ѧ��������õ���Ar����Ԫ�ط��ű�ʾ����ͬ������������ǿ����Na�������µ���ΪҺ̬�ķǽ���Ԫ����Br�����ڹ���Ԫ�ص���K���ÿ�����ĸ��ʾ��������������ˮ�����������ǿ�Ļ�������HClO4�������ڱ�������λ�ã��������ڢ��壮
��2��D��F��B��̬�⻯������H2O���ȶ����ѧʽ����B������⻯��ĵ���ʽ
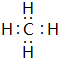 ����ռ乹��Ϊ�������壮
����ռ乹��Ϊ�������壮��3������������ԭ�Ӱ뾶��С����Cl��д��BD2���������������������ۼ���
��4��B��E������������Ӧ��ˮ���������Խ�ǿ����H2SO4�����ѧʽ����д��������֤�ý��۵�һ�����ӷ�Ӧ����ʽCO32-+2H+=CO2��+H2O��HCO3-+H+=CO2��+H2O��
��5����ˮ���ܽⲻ�������ᷴӦ�Ľ�����Au��Pt�ȣ���ԭ������ˮ�в�������HNO3�����з�Ӧ���ɵ�Cl2��NOCl������NOCl������ԭ�Ӷ��ﵽ��8�����ȶ��ṹ����д��NOCl�ĵ���ʽ
 ��
����6��EBC-��A2D2��Ӧ������ED42-�ͿɲμӴ���ѭ���������������壬�Լ�ˮ�������ӣ���д���÷�Ӧ�����ӷ���ʽ2SCN-+11H2O2=2SO42-+2CO2��+N2��+10H2O+2H+��
���� ͼΪ���ڱ���ǰ�����ڣ���Ԫ�������ڱ���λ�ÿ�֪��AΪ�⡢BΪ̼��CΪ����DΪ����EΪ��FΪCl��GΪAr��HΪNa��IΪMg��JΪBr��KΪTi��
��1��ϡ�����������Ϊ�ȶ��ṹ����ѧ��������ã�ͬ����������ҽ����Լ�����ͬ�������϶��½�������ǿ�������µ���ΪҺ̬�ķǽ���Ԫ�����壻����K���ڹ���Ԫ�أ�����������ˮ�����������ǿ�Ļ������Ǹ����ᣬ�����ڱ�������λ��Ϊ�������ڢ��壻
��2���ǽ�����Խǿ�����⻯��Խ�ȶ��ԣ�B������⻯��ΪCH4��
��3��ͬ������ԭ����������ԭ�Ӱ뾶��С��CO2��������̼ԭ������ԭ��֮���γɹ��ۼ���
��4��B��E������������Ӧ��ˮ����ֱ�Ϊ̼�ᡢ���ᣬ�������Ա�̼��ǿ����������ǿ���Ʊ�����ԭ��������֤��
��5��NOCl������ԭ�Ӷ��ﵽ��8�����ȶ��ṹ���γ�8���ӽṹ��N��O��Clԭ�ӷֱ���Ҫ������Ϊ3��2��1����Nԭ����Clԭ��֮���γ�1�Թ��õ��Ӷԣ�Nԭ����Oԭ��֮���γ�2�Թ��õ��Ӷԣ�
��6��EBC-ΪSCN-��A2D2ΪH2O2�����߷�Ӧ����SO42-�ͿɲμӴ���ѭ���������������壬�Լ�ˮ�������ӣ���������Ϊ������̼��������
��� �⣺ͼΪ���ڱ���ǰ�����ڣ���Ԫ�������ڱ���λ�ÿ�֪��AΪ�⡢BΪ̼��CΪ����DΪ����EΪ��FΪCl��GΪAr��HΪNa��IΪMg��JΪBr��KΪTi��
��1��ϡ������Ar�����Ϊ�ȶ��ṹ����ѧ��������ã�ͬ����������ҽ����Լ�����ͬ�������϶��½�������ǿ��������Ԫ����Na�Ľ�������ǿ�������µ���ΪҺ̬�ķǽ���Ԫ�����壻����K���ڹ���Ԫ�أ�����������ˮ�����������ǿ�Ļ������Ǹ����ᣬ��ѧʽΪHClO4�������ڱ�������λ��Ϊ�������ڢ��壬
�ʴ�Ϊ��Ar��Na��Br��K��HClO4���������ڢ��壻
��2���ǽ�����O��Cl��C���ǽ�����Խǿ�����⻯��Խ�ȶ��ԣ���H2O���ȶ���B������⻯��ΪCH4������ʽΪ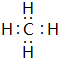 ���ռ乹��Ϊ�������壬
���ռ乹��Ϊ�������壬
�ʴ�Ϊ��H2O��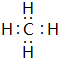 ���������壻
���������壻
��3��ͬ������ԭ����������ԭ�Ӱ뾶��С���ʵ���������Clԭ�Ӱ뾶��С��ϡ������Ԫ�ذ뾶��ͬ����±��ԭ�Ӵ�����������ͬ��һ�㲻�Ƚϣ���CO2��������̼ԭ������ԭ��֮���γɹ��ۼ���
�ʴ�Ϊ��Cl�����ۼ���
��4��B��E������������Ӧ��ˮ����ֱ�Ϊ̼�ᡢ���ᣬ�������Ա�̼��ǿ����������ǿ���Ʊ�����ԭ��������֤����Ӧ�����ӷ�Ӧ����ʽΪ��CO32-+2H+=CO2��+H2O��HCO3-+H+=CO2��+H2O��
�ʴ�Ϊ��H2SO4��CO32-+2H+=CO2��+H2O��HCO3-+H+=CO2��+H2O��
��5��NOCl������ԭ�Ӷ��ﵽ��8�����ȶ��ṹ���γ�8���ӽṹ��N��O��Clԭ�ӷֱ���Ҫ������Ϊ3��2��1����Nԭ����Clԭ��֮���γ�1�Թ��õ��Ӷԣ�Nԭ����Oԭ��֮���γ�2�Թ��õ��Ӷԣ������ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��6��EBC-ΪSCN-��A2D2ΪH2O2�����߷�Ӧ����SO42-�ͿɲμӴ���ѭ���������������壬�Լ�ˮ�������ӣ���������Ϊ������̼���������÷�Ӧ���ӷ���ʽΪ��2SCN-+11H2O2=2SO42-+2CO2��+N2��+10H2O+2H+��
�ʴ�Ϊ��2SCN-+11H2O2=2SO42-+2CO2��+N2��+10H2O+2H+��
���� ���⿼��Ԫ�����ڱ���Ԫ���������ۺ�Ӧ�ã��Ƕ�ѧ���ۺ������Ŀ��飬��Ҫѧ���߱���ʵ�Ļ�����ע���Ԫ�������ɵ��������գ��Ѷ��еȣ�

| A�� | ���������� | B�� | һ�������ж���������ԭ�� | ||
| C�� | ���������� | D�� | ���ǻ����� |
| A�� | H2O | B�� | O3 | C�� | H2O2 | D�� | HClO |
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | �� | |||
| 4 | �� | �� |
��2��Ԫ�آܵ����ӽṹʾ��ͼΪ
 ��
����3���١��ࡢ������Ԫ�ص������Ӱ뾶�ɴ�С��˳���ǣ�����Ӧ�����ӷ��ű�ʾ����Cl-��F-��Na+
��4��д���ܺ͢��γɵĻ�����ĵ���ʽ��

��5�������
�������۵ĵ����û����ĵ��ʵĻ�ѧ����ʽ2Mg+CO2$\frac{\underline{\;��ȼ\;}}{\;}$2MgO+C
�������ٺ͢�����Ԫ������������Ӧ��ˮ�������Ӧ�Ļ�ѧ����ʽΪ��NaOH+Al��OH��3=NaAlO2+2H2O
�������ں͢�����Ԫ������������Ӧ��ˮ�������Ӧ�����ӷ���ʽΪ��H++OH-=H2O
�������ٵ�����������Ӧˮ�����ˮ��Һ��ݵ���������ﷴӦ�Ļ�ѧ����ʽΪAl2O3+2NaOH=2NaAlO2+H2O��
| A�� | ��ѧ��ֻ�����ڷ����ڣ����Ӽ�������ֻ�����ڷ��Ӽ� | |
| B�� | ���Ӿ������ۻ�ʱ�����ۼ�û�б��ƻ� | |
| C�� | �ھ������������Ӵ���ʱ����һ���������Ӵ��� | |
| D�� | ����������ˮ�Ĺ����У���ѧ��һ���ᱻ�ƻ���ı� |
��пƬ�ܽ�32.5g ��пƬ����32.5g ��ͭƬ������1gH2 ��ͭƬ������1mol H2��
| A�� | �٢� | B�� | �٢� | C�� | �ڢ� | D�� | �ڢ� |
 A��B��C��D��E��F��G�Ǻ˵������������Ķ���������Ԫ�أ�AԪ��ԭ�ӵĺ�������������Ӳ�������������������ȣ�Ԫ��B��һ�ֳ������ʿ������Ե缫���ϣ�C��D��G����Ԫ�������ڱ��е����λ����ͼ����ʾ��C��D��F��������֮�͵���E��G��������֮�ͣ�E��FΪ����Ԫ�أ�ֻ��GԪ�صĵ�������ˮ��Ӧ���������ᣮ�ס��ҡ�M��W��X��Y��Z�������ʾ���A��C��D����Ԫ���е�һ�ֻ�����ɣ�����ֻ��M����ͬʱ��������Ԫ�أ�WΪA��C��Ԫ����ɵ�18���ӷ��ӣ��������ȼ�ϣ��ס���Ϊ�ǽ������ʣ�����֮���ת����ϵ��ͼ����ʾ��
A��B��C��D��E��F��G�Ǻ˵������������Ķ���������Ԫ�أ�AԪ��ԭ�ӵĺ�������������Ӳ�������������������ȣ�Ԫ��B��һ�ֳ������ʿ������Ե缫���ϣ�C��D��G����Ԫ�������ڱ��е����λ����ͼ����ʾ��C��D��F��������֮�͵���E��G��������֮�ͣ�E��FΪ����Ԫ�أ�ֻ��GԪ�صĵ�������ˮ��Ӧ���������ᣮ�ס��ҡ�M��W��X��Y��Z�������ʾ���A��C��D����Ԫ���е�һ�ֻ�����ɣ�����ֻ��M����ͬʱ��������Ԫ�أ�WΪA��C��Ԫ����ɵ�18���ӷ��ӣ��������ȼ�ϣ��ס���Ϊ�ǽ������ʣ�����֮���ת����ϵ��ͼ����ʾ��| C | D | |
| G |
��1��Z�Ļ�ѧʽΪNO2���ҵĽṹʽΪN��N��
��2��B�����������ĵ���ʽΪ
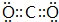 ��
����3���õ���ʽ��ʾAԪ�غ�EԪ���γɻ�����Ĺ��̣�
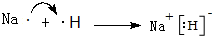 ��
����4��G�ĵ�����ˮ��Ӧ�����ӷ���ʽCl2+H2O?H++Cl-+HClO��
��5��F�ĵ�����E������������ˮ���ﷴӦ�����ӷ���ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2����
��6��W����ȼ�ϵ����һ�ּ���ȼ�ϵ�أ��������Һ��20%��30%��KOH��Һ��W����ȼ�ϵ�طŵ�ʱ������ӦʽΪN2H4+4OH--4e-=N2��+4H2O��
