��Ŀ����
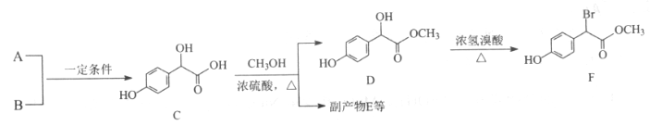
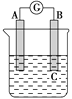
����Ŀ��ij��ѧ��ȤС������ͼ��ʾװ��̽��������ķ�Ӧԭ����

��ش��������⣺
(1) ����M������Ϊ__________��
(2) װ�â�����˿���������⣬���ɿ��Ʒ�Ӧ�Ľ��У�ʹ��Ӧֹͣ�IJ�����__________��
(3)װ�â��������屽�Ļ�ѧ����ʽΪ______________________________��
(4)�����ܵĽ�ˮ��Ϊ________��(����a������b��������ͬ����������_____________��
(5)����֤����Һ�巢������ȡ����Ӧ��������______________��
(6)װ�â��У�����ȥװ�б���С�Թܣ�������ֱ�Ӳ�����������Һ����ʵ��Ŀ�Ĵ�ɻ����ʲôӰ��__________________________
(7)װ��III�е����ӷ�Ӧ����ʽΪ_______________________________________��
���𰸡���Һ©�� ����˿�γ�Һ�� ![]() +Br2
+Br2![]() +HBr a ������������Һ�壬����ԭ����ʧ�� ��Һ����dz��ɫ�������� û�б���������������ҺҲ�����dz��ɫ��������ȷ��HBr���� H+ + OH- = H2O
+HBr a ������������Һ�壬����ԭ����ʧ�� ��Һ����dz��ɫ�������� û�б���������������ҺҲ�����dz��ɫ��������ȷ��HBr���� H+ + OH- = H2O
��������
����ʵ��Ŀ�����Ʊ��屽��ԭ����![]() +Br2
+Br2![]() +HBr����Ϊ����Һ���ӷ������HBr�л���һ���ֱ��������������������HBr�ļ���������ţ������ȥ��һ��ע��β���Ĵ�����
+HBr����Ϊ����Һ���ӷ������HBr�л���һ���ֱ��������������������HBr�ļ���������ţ������ȥ��һ��ע��β���Ĵ�����
��1����������M���ص㣬�Ƴ�����MΪ��Һ©����
��2����˿�ڷ�Ӧ����������������˿���뵽���Һ�У���Ӧ���У�����˿�γ�����Ӧֹͣ��
��3������Һ��������FeBr3�������·���ȡ����Ӧ����Ӧ����ʽΪ![]() +Br2
+Br2![]() +HBr��
+HBr��
��4��Ϊ����ǿ����Ч��������ˮ���½��ϳ�������a�ڽ�ˮ������Һ�巴Ӧ�Ƿ��ȷ�Ӧ���ұ���Һ���ӷ�����������ܵ�������������������Һ�壬����ԭ����ʧ�����ԭ�ϵ������ʣ�
��5��֤������Һ�巢��ȡ����Ӧ����Ҫ��֤HBr������������Һ�г���dz��ɫ������
��6������Һ���ӷ����ӷ�����HBr�л�����������������������������Һ��Ӧ����dz��ɫ��������HBr�ļ���������ţ������������dz�ȥHBr�������������ֱ�Ӳ�����������Һ����ɣ�û�б���������������Һ��Ҳ�����dz��ɫ��������ȷ��HBr�Ĵ��ڣ�
��7����װ�õ�������β��������HBr�Ի�������Ⱦ�������ȥ���������ӷ���ʽΪH����OH��=H2O��