��Ŀ����
��9�֣�����֬�ް�סԼ0��2g�������Ʒ�ĩ������ʯ�����ϣ�����֬���ϵ�ˮ���۲쵽��֬����ȼ��������
��1��������ʵ���������Ƴ��Ľ����ǣ���һ�����������ɣ��ڶ��� ��
Na2O2��ˮ��Ӧ�Ļ�ѧ����ʽ�� ��
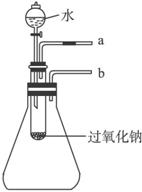
��2��ij�о���ѧϰС��������ͼ��ʾװ�ã����������ã�����ʵ�飬��֤���������ۡ�
��������֤��һ�����۵�ʵ������������ǣ�
��������֤�ڶ������۵�ʵ������������ǣ�
��3��ʵ�飨2�����Թ��м�ˮ��������ȫ�ܽ��Ҳ������������ɺ����̪��Һ��������Һ�ȱ�����ɫ��Ϊ̽����ԭ��С��ͬѧ�Ӳ����й������е�֪��Na2O2��ˮ��Ӧ������H2O2��H2O2����ǿ�����Ժ�Ư���ԡ��������һ���Ƕȣ���������ʹ�����ָʾ�������һ����ʵ�飬��֤Na2O2������ˮ��ַ�Ӧ�����Һ����H2O2���ڡ�
��
��1���÷�Ӧ���ȣ�1�֣� 2Na2O2+2H2O=4NaOH+O2����2�֣�
��2�����ڵ���P���ռ�һ�Թ����壬�������в�������ǵ�ľ��������ʹľ����ȼ����˵��ԭ��Ӧ����O2������2�֣�
���ڵ���Q������һ���������������ͣ���˵��ԭ��Ӧ���ȡ���2�֣�
��3��ȡ������Ӧ�����Һ��һ֧�ྻ���Թ��У�����MnO2����Ѹ�ٷų��������壬��˵��Na2O2������ˮ��ַ�Ӧ�����Һ����H2O2���ڡ���2�֣�
�������е�2��3С�����������𰸾��ɸ��֣�
����:

 ��2008?��ׯһģ����ѧ������ʦ����һ����ʾʵ�飺����֬�ް�סԼ0.2g�������Ʒ�ĩ������ʯ�����ϣ�����֬���ϵ�ˮ���۲쵽��֬����ȼ��������
��2008?��ׯһģ����ѧ������ʦ����һ����ʾʵ�飺����֬�ް�סԼ0.2g�������Ʒ�ĩ������ʯ�����ϣ�����֬���ϵ�ˮ���۲쵽��֬����ȼ�������� ��1������֬�ް�סԼ0.2g�������Ʒ�ĩ������ʯ�����ϣ�����֬���ϵ�ˮ���ɹ۲쵽��֬����ȼ����������ʵ���������ó����й�Na2O2��H2O��Ӧ�Ľ����ǣ�
��1������֬�ް�סԼ0.2g�������Ʒ�ĩ������ʯ�����ϣ�����֬���ϵ�ˮ���ɹ۲쵽��֬����ȼ����������ʵ���������ó����й�Na2O2��H2O��Ӧ�Ľ����ǣ�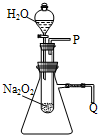 ����֬�ް�סԼ0.2g�������Ʒ�ĩ������ʯ�����ϣ�����֬���ϵ�ˮ���۲쵽��֬����ȼ��������
����֬�ް�סԼ0.2g�������Ʒ�ĩ������ʯ�����ϣ�����֬���ϵ�ˮ���۲쵽��֬����ȼ��������