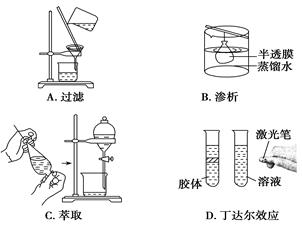
��Ŀ����
(14�֣���ŨCaCl2��Һ��ͨ��NH3��CO2�������Ƶ�����̼��ƣ�����ֱ����1~100nm֮�䣩����ͼ��ʾA~EΪʵ���ҳ���������װ�ã����̶ֹ��г�װ����ȥ���������Ҫ��ش����⡣
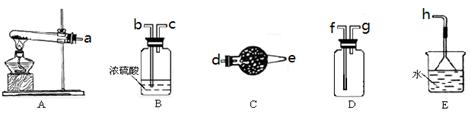
��1��ʵ������ȡ���ռ������NH3����ѡ����������װ�õĽӿ�����˳���ǣ�ѡ ����ĸ����a�� �� �� �� ��h ����Aװ����ȡNH3�Ļ�ѧ��Ӧ����ʽΪ ��
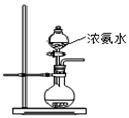
��2����ͼ��ʾװ��Ҳ������ȡNH3����Բ����ƿ�еĹ������ѡ�� ��ѡ����ĸ��ţ���
E���ռ�
��3����ŨCaCl2��Һ��ͨ��NH3��CO2����������̼���ʱ��Ӧ��ͨ��������� ����д��������̼��ƵĻ�ѧ����ʽ ��
��4������Ƽ�ʵ�鷽�����ж�����̼�����Ʒ�����Ƿ�Ϊ���� ��

��1��ʵ������ȡ���ռ������NH3����ѡ����������װ�õĽӿ�����˳���ǣ�ѡ ����ĸ����a�� �� �� �� ��h ����Aװ����ȡNH3�Ļ�ѧ��Ӧ����ʽΪ ��
��2����ͼ��ʾװ��Ҳ������ȡNH3����Բ����ƿ�еĹ������ѡ�� ��ѡ����ĸ��ţ���

| A����ʯ�� | B����ʯ�� | C����ˮ�Ȼ��� | D����ˮ����ͭ |
��3����ŨCaCl2��Һ��ͨ��NH3��CO2����������̼���ʱ��Ӧ��ͨ��������� ����д��������̼��ƵĻ�ѧ����ʽ ��
��4������Ƽ�ʵ�鷽�����ж�����̼�����Ʒ�����Ƿ�Ϊ���� ��
��1��d e g f��2�֣�2NH4Cl+Ca(OH)2 CaCl2 + 2NH3 ��+ H2O�������𰸺���Ҳ���֣���2�֣�
CaCl2 + 2NH3 ��+ H2O�������𰸺���Ҳ���֣���2�֣�
��2��ABE��3��©ѡһ����1�֣���ѡһ������1�֣�
��3��NH3��2�֣� CaCl2 + CO2 + 2NH3 + H2O��CaCO3�� + 2NH4Cl��2�֣�
��4��ȡ������Ʒ��ˮ����γɷ�ɢϵ����һ�������䣬������һ��������ͨ·����������̼��ƣ������ǣ�3�֣�
 CaCl2 + 2NH3 ��+ H2O�������𰸺���Ҳ���֣���2�֣�
CaCl2 + 2NH3 ��+ H2O�������𰸺���Ҳ���֣���2�֣���2��ABE��3��©ѡһ����1�֣���ѡһ������1�֣�
��3��NH3��2�֣� CaCl2 + CO2 + 2NH3 + H2O��CaCO3�� + 2NH4Cl��2�֣�
��4��ȡ������Ʒ��ˮ����γɷ�ɢϵ����һ�������䣬������һ��������ͨ·����������̼��ƣ������ǣ�3�֣�
��1��ʵ��������ʯ�Һ��Ȼ�識�����ȡ����������A�Ƿ���װ�ã���Ӧ�ķ���ʽΪ2NH4Cl+Ca(OH)2 CaCl2 + 2NH3 ��+ H2O�������Ǽ������壬Ӧ���ü�ʯ�Ҹ��ﰱ����������Ũ���ᡣ�������ܶ�С�ڿ����ģ��Ұ�����������ˮ������Ӧ���������ſ������ռ�����������˳����d e g f��
CaCl2 + 2NH3 ��+ H2O�������Ǽ������壬Ӧ���ü�ʯ�Ҹ��ﰱ����������Ũ���ᡣ�������ܶ�С�ڿ����ģ��Ұ�����������ˮ������Ӧ���������ſ������ռ�����������˳����d e g f��
��2����ˮ�д���ƽ��NH3��H2O NH3��H2O
NH3��H2O NH4����OH��������Ҫ��ȡ��������Ӧ��ʱƽ�����淴Ӧ�����ƶ������Դ�ѡABE��
NH4����OH��������Ҫ��ȡ��������Ӧ��ʱƽ�����淴Ӧ�����ƶ������Դ�ѡABE��
��3��CO2��ˮ��Һ�е��ܽ��С����������������ˮ����������ͨ����ǰ�������Ӧ�ķ���ʽΪCaCl2 + CO2 + 2NH3 + H2O��CaCO3�� + 2NH4Cl��
��4����������ֱ���������ģ������γɵķ�ɢ��Ӧ���ǽ��壬����������ö����ЧӦ����ȡ������Ʒ��ˮ����γɷ�ɢϵ����һ�������䣬������һ��������ͨ·����������̼��ƣ������ǡ�
 CaCl2 + 2NH3 ��+ H2O�������Ǽ������壬Ӧ���ü�ʯ�Ҹ��ﰱ����������Ũ���ᡣ�������ܶ�С�ڿ����ģ��Ұ�����������ˮ������Ӧ���������ſ������ռ�����������˳����d e g f��
CaCl2 + 2NH3 ��+ H2O�������Ǽ������壬Ӧ���ü�ʯ�Ҹ��ﰱ����������Ũ���ᡣ�������ܶ�С�ڿ����ģ��Ұ�����������ˮ������Ӧ���������ſ������ռ�����������˳����d e g f����2����ˮ�д���ƽ��NH3��H2O
 NH3��H2O
NH3��H2O NH4����OH��������Ҫ��ȡ��������Ӧ��ʱƽ�����淴Ӧ�����ƶ������Դ�ѡABE��
NH4����OH��������Ҫ��ȡ��������Ӧ��ʱƽ�����淴Ӧ�����ƶ������Դ�ѡABE����3��CO2��ˮ��Һ�е��ܽ��С����������������ˮ����������ͨ����ǰ�������Ӧ�ķ���ʽΪCaCl2 + CO2 + 2NH3 + H2O��CaCO3�� + 2NH4Cl��
��4����������ֱ���������ģ������γɵķ�ɢ��Ӧ���ǽ��壬����������ö����ЧӦ����ȡ������Ʒ��ˮ����γɷ�ɢϵ����һ�������䣬������һ��������ͨ·����������̼��ƣ������ǡ�

��ϰ��ϵ�д�
 ͬ����ϰ����ʦ����ѧ������ϵ�д�
ͬ����ϰ����ʦ����ѧ������ϵ�д� ����ϰ�⽭��ϵ�д�
����ϰ�⽭��ϵ�д� ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�
�����Ŀ