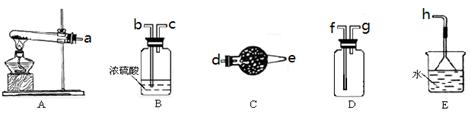
��Ŀ����
��ҵ���õ�ⱥ��ʳ��ˮ�ķ�����ȡ�������ơ������������������ǰҪ���д��ξ��ơ��Իش��������⣺
���Ͽ�Ƭ��

(1)ѡ���Լ��Գ�ȥ�±����еĿ��������ʡ�
(2)���ƹ��̼�Ҫ����������Ca2����Mg2����SO42�� ��ȫ��������Ҫ��֤�������µ����ʣ�Ϊ������Ϊ������ѡ�������ĺ���˳��Ϊ����________��________(�����)��
(3)���������������Ե�����Һ�����Բ���ȥ������CO32��.���������ȥCO32���Ļ�ѧ����ʽΪ______________________________________________________________________��
���Ͽ�Ƭ��

(1)ѡ���Լ��Գ�ȥ�±����еĿ��������ʡ�
| ���� | ������Լ� |
| CaCl2 | ��________ |
| MgCl2 | ��________ |
| ������ | ��________ |
(3)���������������Ե�����Һ�����Բ���ȥ������CO32��.���������ȥCO32���Ļ�ѧ����ʽΪ______________________________________________________________________��
��1����.Na2CO3 ��.NaOH ��.BaCl2 ��2����
��3��Na2CO3+2HCl=2NaCl+CO2��+H2O
��3��Na2CO3+2HCl=2NaCl+CO2��+H2O
��1�����ڲ����������µ����ʣ����Կ���̼����������ȥCa2������������������ȥMg2�����Ȼ���������ȥSO42����
��2�����ڹ������Ȼ�����Ҫͨ��̼��������ȥ�����̼���Ʊ�������Ȼ����ĺ��棬����ȷ˳���Ǣ�
��3�����������ǿ��̼��ģ����������̼���Ʒ�Ӧ�ķ���ʽΪ
Na2CO3+2HCl=2NaCl+CO2��+H2O��
��2�����ڹ������Ȼ�����Ҫͨ��̼��������ȥ�����̼���Ʊ�������Ȼ����ĺ��棬����ȷ˳���Ǣ�
��3�����������ǿ��̼��ģ����������̼���Ʒ�Ӧ�ķ���ʽΪ
Na2CO3+2HCl=2NaCl+CO2��+H2O��

��ϰ��ϵ�д�
 53���ò�ϵ�д�
53���ò�ϵ�д�
�����Ŀ