��Ŀ����
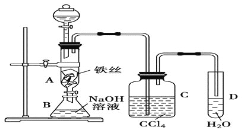
����Ŀ��I.ijͬѧ�����ͼ��ʾװ��̽�������ܷ���ˮ������Ӧ������a����Ҫ�ɷ��Ǻ�������ˮ��������������ش��������⣺
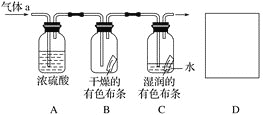
(1) Ũ�����������_____________________________��
(2) ֤��������ˮ������Ӧ��ʵ������Ϊ_________________________��
(3) ��ʵ����ƴ��ڵ�ȱ����____________________��Ϊ�˿˷���ȱ�ݣ���Ҫ����װ��D�����з�����Ӧ�����ӷ���ʽΪ_________________________��
II.��ͼΪŨ������ͭ��Ӧ�����������ʵ��װ��
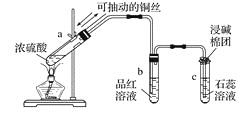
�ش��������⣺
(1) ָ���Թ�b��c�в�����ʵ������b��___________________��c��______________________��
(2) ��Ӧ�������Թ�a���в��ְ�ɫ���壬��a�Թ��е���Һ��������ˮ�У������ı仯��___________��
(3) д��Ũ������ͭ��Ӧ�Ļ�ѧ����ʽ��_____________________________��
���𰸡���ȥ�����е�ˮ�����������������װ��B�е���ɫ��������ɫ��װ��C�е���ɫ������ɫû��β������װ��Cl2+2OH-=Cl-+ClO-+H2OƷ����Һ��ɫʯ����Һ���ɫ��ɫ�����ܽ⣬��Һ�����ɫCu+2H2SO4��Ũ��![]() CuSO4+SO2��+2H2O
CuSO4+SO2��+2H2O
��������
I.(1)Ũ���������ˮ�ԣ����������������ʵ������������a�е�ˮ�������ʴ�Ϊ����ȥ�����е�ˮ����(���������)��
(2)��������������Ư���ԣ�������ˮ��Ӧ��������ʹ����ᣬ����ʽΪ��Cl2+H2O=HCl+HClO�����������Ư���ԣ����֤��������ˮ������Ӧ��ʵ������Ϊװ��B�е���ɫ��������ɫ��װ��C�е���ɫ������ɫ���ʴ�Ϊ��װ��B�е���ɫ��������ɫ��װ��C�е���ɫ������ɫ��
(3)�����ж���Ӧ����β�����������������ŷŵ������У����ü���Һ�����գ�Cl2+2NaOH=NaCl+NaClO+H2O�����ӷ���ʽΪ��Cl2+2OH-=Cl-+ClO-+H2O���ʴ�Ϊ��û��β������װ�ã�Cl2+2OH-=Cl-+ClO-+H2O��
II. Ũ������ͭ�ڼ������������ɷ�ӦCuSO4��SO2��H2O��Ӧ����ʽΪ��Cu+2H2SO4(Ũ)![]() CuSO4+SO2��+2H2O��
CuSO4+SO2��+2H2O��
(1)�����������Ư���ԣ��ܹ�ʹƷ����Һ��ɫ�����������������������ˮ��Һ�����ԣ��ܹ�ʹʯ����Һ���ɫ���ʴ�Ϊ��Ʒ����Һ��ɫ��ʯ����Һ���ɫ��
(2) ��Ӧ�������Թ�a���в��ְ�ɫ����Ϊ��ˮ����ͭ����a�Թ��е���Һ��������ˮ�У�����ͭ����ˮ����Һ�����ɫ���ʴ�Ϊ����ɫ�����ܽ⣬��Һ�����ɫ��
(3)Ũ������ͭ�ڼ������������ɷ�ӦCuSO4��SO2��H2O��Ӧ����ʽΪ��Cu+2H2SO4(Ũ)![]() CuSO4+SO2��+2H2O���ʴ�Ϊ��Cu+2H2SO4(Ũ)
CuSO4+SO2��+2H2O���ʴ�Ϊ��Cu+2H2SO4(Ũ)![]() CuSO4+SO2��+2H2O��
CuSO4+SO2��+2H2O��