��Ŀ����
��14�֣���ϸͭ����ҪӦ���ڵ�����ϡ������������С���ϸͭ�۵�ij�Ʊ��������£�
�Իش��������⣺
��1�����й���[Cu(NH3)4]SO4��˵���У���ȷ����__________��������ĸ��ţ�
A��[Cu(NH3)4]SO4�������Ļ�ѧ�������Ӽ������Լ�����λ��
B��[Cu(NH3)4]SO4����NH3���ӣ���ˮ��Һ��Ҳ����NH3����
C��[Cu(NH3)4]SO4�����Ԫ���е�һ��������������Ԫ��
D��[Cu(NH3)4]SO4��������ӵĿռ乹��Ϊ��������
��2��NH4CuSO3�еĽ��������ӵĺ�������Ų�ʽΪ_______________��
��3��SO2�C3 ������S��ԭ�ӵ��ӻ���ʽΪ________�����以Ϊ�ȵ������һ�ַ��ӵķ���ʽ��___________��
��4��NH3��Һ����ԭ����___________________��
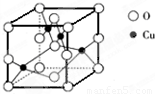
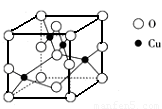
��5����ͼ��ͭ��ij��������ľ����ṹʾ��ͼ���ɴ˿�ȷ����������Ļ�ѧʽ_____��
��6��NH4CuSO3�������ȷ�Ӧ�����ӷ���ʽΪ__________________________��
��1�� AD��2�֣�
��2�� ��Ar��3d10��2�֣�
��3�� sp3��NF3����PF3��NCl3��PCl3�ȣ��� ��2�֣�
��4�� NH3���Ӽ���γ��������2�֣�
��5�� CuO��2�֣�
��6��2NH4CuSO3
+ 4H+  2NH4+ + Cu2+
+ Cu + 2SO2�� + 2H2O��2�֣�
2NH4+ + Cu2+
+ Cu + 2SO2�� + 2H2O��2�֣�
����������

 ����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
����ν����Ž̲��㽭���̴�ѧ������ϵ�д� �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д���14�֣���ϸͭ����ҪӦ���ڵ�����ϡ������������С���ϸͭ�۵�ij�Ʊ��������£�
�Իش��������⣺
��1�����й���[Cu(NH3)4]SO4��˵���У���ȷ����__________��������ĸ��ţ�
| A��[Cu(NH3)4]SO4�������Ļ�ѧ�������Ӽ������Լ�����λ�� |
| B��[Cu(NH3)4]SO4����NH3���ӣ���ˮ��Һ��Ҳ����NH3���� |
| C��[Cu(NH3)4]SO4�����Ԫ���е�һ��������������Ԫ�� |
| D��[Cu(NH3)4]SO4��������ӵĿռ乹��Ϊ�������� |
��3��SO2�C3 ������S��ԭ�ӵ��ӻ���ʽΪ________�����以Ϊ�ȵ������һ�ַ��ӵķ���ʽ��___________��
��4��NH3��Һ����ԭ����___________________��
��5����ͼ��ͭ��ij��������ľ����ṹʾ��ͼ���ɴ˿�ȷ����������Ļ�ѧʽΪ__
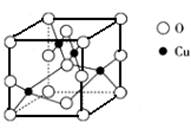 ___________��
___________����6��NH4CuSO3�������ȷ�Ӧ�����ӷ���ʽΪ__________________________��
 ��ϸͭ����ҪӦ���ڵ�����ϡ������������У���ϸͭ�۵�ij�Ʊ��������£�
��ϸͭ����ҪӦ���ڵ�����ϡ������������У���ϸͭ�۵�ij�Ʊ��������£�

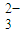 ������S��ԭ�ӵ��ӻ���ʽΪ______�����以Ϊ�ȵ������һ�ַ��ӵķ���ʽ��______��
������S��ԭ�ӵ��ӻ���ʽΪ______�����以Ϊ�ȵ������һ�ַ��ӵķ���ʽ��______��