��Ŀ����
�����£�0.1 mol��L��1��HA��Һ��c(OH��)/c(H��)��1��10��8��������������ȷ����
A��0.01 mol��L��1HA����Һ��c(H��)��1��10��4mol��L��1
B��pH��3��HA��Һ��pH��11��NaOH��Һ�������Ϻ�������Һ��c(Na��)��c(A��)��c(OH��)��c(H��)
C��Ũ�Ⱦ�Ϊ0.1 mol��L��1��HA��Һ��NaA��Һ�������Ϻ�������Һ�����ԣ���c(OH��)��c(H��)��c(HA)��c(A��)
D��pH��3��HA��Һ��pH��11��NaOH��Һ�������1?10��Ϻ�������Һ��c(OH��)��c(A��)��c(H��)��c(Na��)
D
����

 ��ѧȫ��������ѵ��ϵ�д�
��ѧȫ��������ѵ��ϵ�д����й��ڵ������Һ��������ȷ����( )
| A�������£���pH��7�Ĵ����ƺʹ�������Һ�У�c(CH3COO��)��c(Na��) |
| B��ϡ�ʹ�����Һ����Һ���������ӵ�Ũ�Ⱦ����� |
| C����pH��5���Ȼ��ƺ�ϡ����Ļ����Һ�У�c(Na��)��c(Cl��) |
| D��0.1 mol��L��1��������Һ�У�c(OH��)��c(H��)��c(HS��)��c(H2S) |
�����£������е������Һ���й�˵��һ����ȷ����
| A����ͬŨ�Ⱥ������ǿ���ǿ����Һ��Ϻ���Һ��pH��7 |
B����NaHCO3��Һ�У�c( )��c( )��c(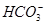 ) ) |
| C������AgCl��������Һ�м���NaCl���壬c(Ag��)��С |
| D����pH��ȵ�CH3COONa��Na2CO3��Һϡ����ͬ������CH3COONa��Һ��pH�ϴ� |
�����£���20.00 mL 0.100 0 mol��L��1CH3COONa��Һ����μ���0.100 0 mol��L��1���ᣬ��Һ��pH��������������Ĺ�ϵ��ͼ��ʾ(�����ǻӷ�)������˵����ȷ���� 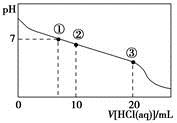
| A�������ʾ��Һ�У�c(CH3COOH)��c(Cl��)>c(OH��)��c(H��) |
| B�������ʾ��Һ�У�c(Na��)>c(Cl��)>c(CH3COO��)>c(CH3COOH) |
| C�������ʾ��Һ�У�c(Na��)>c(CH3COOH>c(CH3COO��) )>c(H��) |
| D�����������п��ܳ��֣�c(H��)��c(Na��)��c(CH3COOH)��c(CH3COO��) |
25��ʱ����Ũ��Ϊ0.1000 mol��L��1��NaOH��Һ�ζ�20.00 mLŨ�Ⱦ�Ϊ0.1000 mol��L��1��������HX��HY��HZ���ζ���������ͼ��ʾ������˵����ȷ����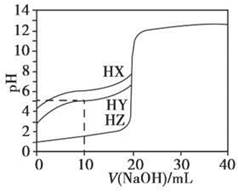
| A������ͬ�¶��£�ͬŨ�ȵ���������Һ�ĵ�������˳��HZ��HY��HX |
| B�����ݵζ����ߣ��ɵ�Ka(HY)��10��5 |
| C��������HX��HY��Һ�������Ϻ���NaOH��Һ�ζ���HXǡ����ȫ��Ӧʱ��c(X��)��c(Y��)��c(OH��)��c(H��) |
D��HY��HZ��ϣ��ﵽƽ��ʱ��c(H��)�� ��c(Z��)��c(OH��) ��c(Z��)��c(OH��) |
�������ʵ���Ũ�ȹ�ϵ�������
| A�������ʵ���Ũ�ȵ�HA��Һ��MOH��Һ�������ϣ�c(H��)��c(M��)��c(OH��)��c(A��) |
| B��pH��ȵ�CH3COONa��NaOH��Na2CO3������Һ��c(NaOH)<c(Na2CO3)<c(CH3COONa) |
| C�����ʵ���Ũ����ȵ�CH3COOH��CH3COONa��Һ�������ϣ� c(CH3COO��)��2c(OH��)��2c(H��)��c(CH3COOH) |
D��0.1 mol��L��1��NaHCO3��Һ��c(Na��)>c(OH��)>c( )>c(H��) )>c(H��) |
��֪���±�Ϊ25 ��ʱijЩ����ĵ���ƽ�ⳣ����ͼ���ʾ�����£�ϡ��CH3COOH��HClO�������ϡ��Һʱ����ҺpH���ˮ���ı仯������˵����ȷ����
| CH3COOH | HClO | H2CO3 |
| Ka��1.8��10��5 | Ka��3.0��10��8 | Ka1��4.4��10��7 Ka2��4.7��10��11 |

A����ͬŨ�ȵ�CH3COONa��NaClO�Ļ��Һ�У�������Ũ�ȵĴ�С��ϵ��
c(Na��)>c(ClO��)>c(CH3COO��)>c(OH��)>c(H��)
B����NaClO��Һ��ͨ������������̼�����ӷ���ʽΪ2ClO����CO2��H2O=2HClO��
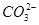
C��ͼ����a��c���㴦����Һ��
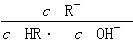 ���(HR����CH3COOH��HClO)
���(HR����CH3COOH��HClO)D��ͼ����a�������Ũ�ȴ���b�������Ũ��
ij����С��Ϊ̽��BaSO4���ܽ�ȣ��ֱ�����BaSO4���룺
��5 mLˮ
��20 mL 0.5 mol��L��1��Na2SO4��Һ
��40 mL 0.2 mol��L��1��Ba(OH)2��Һ
��40 mL 0.1 mol��L��1��H2SO4��Һ�У��ܽ�������
���ϸ���Һ�У�c(Ba2��)�Ĵ�С˳����ȷ����(����)
| A���ۣ��٣��ܣ��� | B���ۣ��٣��ڣ��� |
| C���٣��ܣ��ۣ��� | D���٣��ۣ��ܣ��� |
