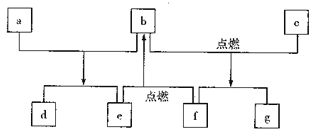
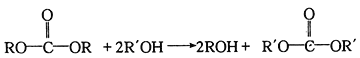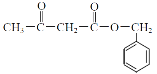��Ŀ����
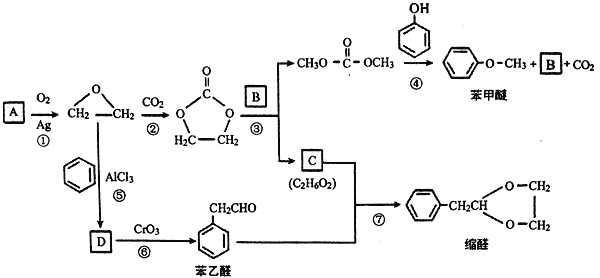
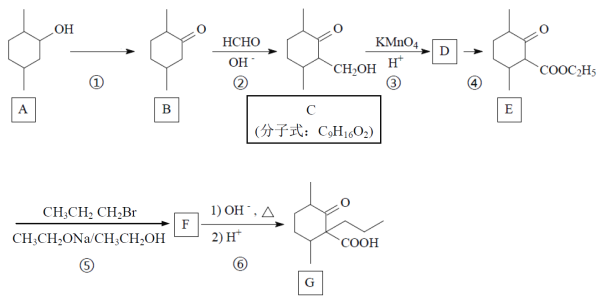
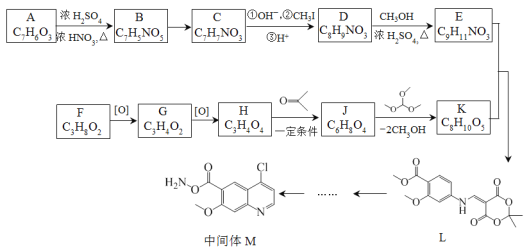
����Ŀ������ҩ���ַ������м���M�ĺϳ�·�����£�
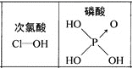
��֪��
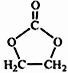
i. ![]()
![]()
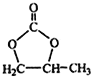
![]() (R��������)
(R��������)
ii. �л���ṹ���ü���ʽ��ʾ����(CH3O)3CH�ļ���ʽΪ![]()
(1)A�к��б���������ȡ����������λ��A�к��еĹ����������� _______��ѪҺ��A��Ũ�ȹ�����ʹ���ж����ɾ�����עNaHCO3��Һ�ⶾ��A��NaHCO3��Ӧ�Ļ�ѧ����ʽ��_______��
(2)B����C�Ĺ����У�B������_____(���������ԭ��)��Ӧ��
(3)D����E�Ļ�ѧ����ʽ��________��
(4)д����������������D������һ��ͬ���칹��Ľṹ��ʽ________��
�ٷ����к���������ֱ�����ڱ�����
�ں˴Ź�������ͼ��ʾ�����������ֻ�ѧ������ͬ����ԭ��
�۲�����FeCl3��Һ������ɫ��Ӧ
(5)F�ĺ˴Ź�������ͼ��������壬�����֮��Ϊ2:1:1��F����G�Ļ�ѧ����ʽ��_____��
(6)H����J�Ļ�ѧ����ʽ��_______��
(7)��֪E+K��L+CH3OH��K�Ľṹ��ʽ��________��
���𰸡��ǻ����Ȼ� ![]() +NaHCO3��
+NaHCO3��![]() +H2O+CO2�� ��ԭ
+H2O+CO2�� ��ԭ ![]() +CH3OH
+CH3OH![]()
![]() +H2O
+H2O 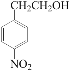 ��
��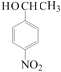 ��
��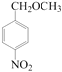 ��
��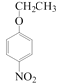 HOCH2CH2CH2OH+O2
HOCH2CH2CH2OH+O2![]() OHCCH2CHO+2H2O
OHCCH2CHO+2H2O ![]() +
+ ![]()
![]()
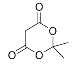 +H2O
+H2O 
��������
�ϳ�E��·��: A����ʽΪC7H6O5�����ݣ�1����Ϣ��A���б���������ȡ������������λ������A�����е�����������ֻ����-OH��-COOH������AΪ![]() ��A��B�DZ����ϵ�������Ӧ������L�Ľṹ��֪��������λ�����Ȼ��Ķ�λ������BΪ��
��A��B�DZ����ϵ�������Ӧ������L�Ľṹ��֪��������λ�����Ȼ��Ķ�λ������BΪ��![]() ���Ա�L��֪��B��C��-NO2�Ļ�ԭ������CΪ:
���Ա�L��֪��B��C��-NO2�Ļ�ԭ������CΪ:![]() ����ӦC��D���Ȱ���Ϣ i.
����ӦC��D���Ȱ���Ϣ i. ![]()
![]()
![]() (�˴�R����Ϊ-CH3)��ʽ��Ӧ���ٽ������ữ���D��
(�˴�R����Ϊ-CH3)��ʽ��Ӧ���ٽ������ữ���D��![]() ��D����״�����������Ӧ��E��
��D����״�����������Ӧ��E��![]() ��
��
�ϳ�K��·�ߣ�F����ʽΪC3H8O2���ѱ��ͣ���������G��ʧ4��H��������һ��������H����2��O�������С�⣨5����Ϣ��֪��F��G��H�ֱ�ΪHOCH2CH2CH2OH��OHCCH2CHO��![]() ������E�ṹ�ɶ�L���������ߴ��и
������E�ṹ�ɶ�L���������ߴ��и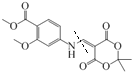 ���ٸ���(7)��Ϣ��E+K��L+CH3OH����K�ķ���ʽ��C8H10O5�������Ƴ�K�Ľṹ��ʽ��
���ٸ���(7)��Ϣ��E+K��L+CH3OH����K�ķ���ʽ��C8H10O5�������Ƴ�K�Ľṹ��ʽ�� ������J�ķ���ʽ��K�Ľṹ�ɶ�K���������ߴ��и
������J�ķ���ʽ��K�Ľṹ�ɶ�K���������ߴ��и ���Ӷ��Ƴ�J�ĽṹΪ��
���Ӷ��Ƴ�J�ĽṹΪ��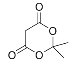 ��
��
�������Ƶ��Ľ�������ϣ��ɶԸ���С����н��
��1���������Ϸ����Ƶ��Ľ����֪��AΪ![]() ����Ϊ����ǿ����ϵΪ���Ȼ�>̼��>���ǻ�������A��NaHCO3��Ӧ�Ļ�ѧ����ʽ�ǣ�
����Ϊ����ǿ����ϵΪ���Ȼ�>̼��>���ǻ�������A��NaHCO3��Ӧ�Ļ�ѧ����ʽ�ǣ�![]() +NaHCO3��
+NaHCO3��![]() +H2O+CO2�����ʴ�Ϊ���ǻ����Ȼ�
+H2O+CO2�����ʴ�Ϊ���ǻ����Ȼ� ![]() +NaHCO3��
+NaHCO3��![]() +H2O+CO2��
+H2O+CO2��
��2��B����C�Ĺ����У�Bʧȥ2��O������2��H������ʧ���ǻ�ԭ��Ӧ���ʴ�Ϊ����ԭ
��3���������Ϸ����Ƶ��Ľ����֪�� DΪ![]() ��EΪ
��EΪ![]() ����ӦΪ��
����ӦΪ��![]() +CH3OH
+CH3OH![]()
![]() +H2O���ʴ�Ϊ��
+H2O���ʴ�Ϊ��![]() +CH3OH
+CH3OH![]()
![]() +H2O
+H2O
��4���������ٷ����к���������ֱ�����ڱ����ϣ����D�Ľṹ![]() ��֪�������ϵ�ȡ������һ�������Ͷȣ������������Ͷ�Ϊ1����������ȡ���������ͣ�
��֪�������ϵ�ȡ������һ�������Ͷȣ������������Ͷ�Ϊ1����������ȡ���������ͣ�
�ٸ��������ڣ��˴Ź�������ͼ��ʾ�����������ֻ�ѧ������ͬ����ԭ�ӣ�
�ۣ͢�������FeCl3��Һ������ɫ��Ӧ�������Ƴ�����������D��ͬ���칹��Ľṹ��ʽΪ�� ��
�� ��
�� ��
�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� ��
�� ��
��
(5) �������Ϸ����Ƶ��Ľ����֪��F��G�ֱ�ΪHOCH2CH2CH2OH��OHCCH2CHO����F����G�Ļ�ѧ����ʽ�ǣ�HOCH2CH2CH2OH+O2![]() OHCCH2CHO+2H2O���ʴ�Ϊ��HOCH2CH2CH2OH+O2
OHCCH2CHO+2H2O���ʴ�Ϊ��HOCH2CH2CH2OH+O2![]() OHCCH2CHO+2H2O
OHCCH2CHO+2H2O
(6) �������Ϸ����Ƶ��Ľ����֪��HΪ![]() ��JΪ
��JΪ  ��H����J�Ļ�ѧ����ʽ�ǣ�
��H����J�Ļ�ѧ����ʽ�ǣ�![]() +
+ ![]()
![]()
 +H2O���ʴ�Ϊ��
+H2O���ʴ�Ϊ��![]() +
+ ![]()
![]()
 +H2O
+H2O
(7) �������Ϸ����Ƶ��Ľ����֪��K�Ľṹ��ʽ�ǣ� ���ʴ�Ϊ��
���ʴ�Ϊ��

 �ľ�ͼ���ʱ�ȷ�ϵ�д�
�ľ�ͼ���ʱ�ȷ�ϵ�д�