��Ŀ����
����Ŀ�������Ǹ�ˮ����[Cr(CH3COO)2]2��2H2O��һ���������ռ���Ϊ����ɫ���壬�ױ�������������ˮ������(�ӷ����л��ܼ�)�������Ҵ������������ᣬ���Ʊ�װ������(��֪Cr3��ˮ��Һ��ɫΪ��ɫ��Cr2��ˮ��Һ��ɫΪ��ɫ)��

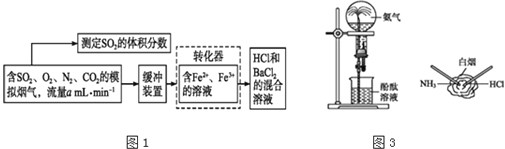
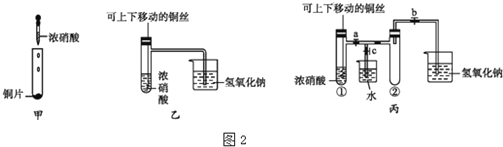
��1��װ�ü�����ͨ��a��������________________________��
��2����������ƿ�����μ������п��������CrCl3��Һ���ر�k2��k1��������Һ©�������������ƺõ��٣����۲쵽������ƿ����Һ��ɫ__________________ʱ����k2�ر�k1�����۲쵽װ�����г��ִ�������ɫ����ʱ���رշ�Һ©����������
��3��װ�����з�Ӧ�����ӷ���ʽΪ__________________________________��
��4����װ�����л������ٹ��ˡ�ϴ�Ӻ�������õ�a g [Cr(CH3COO)2]2��2H2O��ϴ��ʱ������ȥ����������ˮ����ˮ�Ҵ�������ϴ�ӡ������Ҵ�ϴ�ӵ�Ŀ����___________��
��5����ʵ����ȡ�õ�CrCl3��Һ�к�����b g����[Cr(CH3COO)2]2��2H2O (��Է�������Ϊ376) �IJ�����________________��
��6����ʵ��װ����һ�����Ե�ȱ�ݣ������ȱ�ݵĴ�ʩΪ________________________��
���𰸡�ƽ����ѹ�����ڷ�Һ©����Һ����˳������ ��ɫ��ȫ��Ϊ��ɫ 2Cr2+ +4CH3COO- + 2H2O = [Cr(CH3COO)2]2��2H2O ��ȥ�����Ŀ��������ʺ�ˮ�֣����ڿ��ٸ��� ![]() Ӧ��β������ͨ��װ��ˮ��ˮ����
Ӧ��β������ͨ��װ��ˮ��ˮ����
��������
��1��װ�ü�����ͨ��aʹ�����ںͷ�Һ©���Ϸ���ѹǿ��ȣ���ƽ����ѹ�����ã������ڷ�Һ©����Һ����˳�����£�
��2����������ƿ�����μ������п��������CrCl3��Һ���ر�k2��k1��������Һ©�������������ƺõ��٣�������ӦZn+2Cr3+= Zn2++2Cr2+��Zn+2H+= Zn2++H2�������۲쵽������ƿ����Һ��ɫ����ɫ��ȫת��Ϊ��ɫʱ��˵��Cr3+ת��ΪCr2+����k2�ر�k1������ʹ��������ѹǿ���Ѽ�����Һѹ�������������۲쵽װ�����г��ִ�������ɫ����ʱ���رշ�Һ©����������
��3��װ������CrCl2��Һ���������Һ��Ӧ����[Cr(CH3COO)2]2��2H2O��
��4�������Ǹ�ˮ���ﲻ����ˮ�����ѣ������Ҵ�����ˮϴȥ���������ʣ������Ҵ�ϴ�ӣ���ȥ�����Ŀ��������ʺ�ˮ�֣����ڿ��ٸ�������������ϴ�ӽ�һ�����
��5������CrԪ���غ㣬�ɵù�ϵʽ��2CrCl3 ~ [Cr(CH3COO)2]2��2H2O������[Cr(CH3COO)2]2��2H2O�����۲���������������ʣ�
��6�����ڲ�Ʒ�ױ���������˸�ʵ��װ����һ�����Ե�ȱ����װ��β�����п������룬������Ʒ�����Խ�β������ͨ��װ��ˮ��ˮ���з�ֹ�������롣
��1��װ�ü�����ͨ��aʹ�����ںͷ�Һ©���Ϸ���ѹǿ��ȣ���ƽ����ѹ�����ã������ڷ�Һ©����Һ����˳�����£�
�ʴ�Ϊ��ƽ����ѹ�����ڷ�Һ©����Һ����˳�����£�
��2����������ƿ�����μ������п��������CrCl3��Һ���ر�k2��k1��������Һ©�������������ƺõ��٣�������ӦZn+2Cr3+= Zn2++2Cr2+��Zn+2H+= Zn2++H2�������۲쵽������ƿ����Һ��ɫ����ɫ��ȫת��Ϊ��ɫʱ��˵��Cr3+ת��ΪCr2+����k2�ر�k1������ʹ��������ѹǿ���Ѽ�����Һѹ�������������۲쵽װ�����г��ִ�������ɫ����ʱ���رշ�Һ©����������
�ʴ�Ϊ����ɫ��ȫ��Ϊ��ɫ��
��3��װ������CrCl2��Һ���������Һ��Ӧ����[Cr(CH3COO)2]2��2H2O����Ӧ�����ӷ���ʽΪ2Cr2+ +4CH3COO- + 2H2O = [Cr(CH3COO)2]2��2H2O��
�ʴ�Ϊ��2Cr2+ +4CH3COO- + 2H2O = [Cr(CH3COO)2]2��2H2O��
��4�������Ǹ�ˮ���ﲻ����ˮ�����ѣ������Ҵ�����ˮϴȥ���������ʣ������Ҵ�ϴ�ӣ���ȥ�����Ŀ��������ʺ�ˮ�֣����ڿ��ٸ�������������ϴ�ӽ�һ�����
�ʴ�Ϊ����ȥ�����Ŀ��������ʺ�ˮ�֣����ڿ��ٸ��
��5������CrԪ���غ㣬�ɵù�ϵʽ��2CrCl3 ~ [Cr(CH3COO)2]2��2H2O����[Cr(CH3COO)2]2��2H2O�����۲���Ϊ![]() ����[Cr(CH3COO)2]2��2H2O�IJ���Ϊ
����[Cr(CH3COO)2]2��2H2O�IJ���Ϊ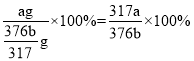 ��
��
�ʴ�Ϊ��![]() ��
��
��6�����ڲ�Ʒ�ױ���������˸�ʵ��װ����һ�����Ե�ȱ����װ��β�����п������룬������Ʒ�������ȱ�ݵĴ�ʩΪ��β������ͨ��װ��ˮ��ˮ���У�
�ʴ�Ϊ��Ӧ��β������ͨ��װ��ˮ��ˮ���С�

 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д� �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д� �������ͬ����ϰϵ�д�
�������ͬ����ϰϵ�д�����Ŀ��I.������ͼ��ʾװ�òⶨ�к��ȵ�ʵ�鲽�����£�
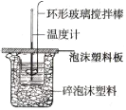
������Ͳ��ȡ50mL0.50mol��L-1���ᵹ��С�ձ��У���������¶ȣ�
������һ��Ͳ��ȡ50mL0.55mol��L-1 NaOH��Һ������ͬһ�¶ȼƲ�����¶ȣ�
�۽�NaOH��Һ����С�ձ��У�ʹ֮��Ͼ��ȣ���û����Һ����¶ȡ�
�ش��������⣺
��1������NaOH��ҺҪ�Թ�����������__��
��2�����β��������������Һ�IJ�����__��
��3��___(������������������)��Ba(OH)2��Һ��H2SO4��Һ����NaOH��Һ�����ᣬ����__��
��4���ֽ�һ������ϡNaOH��Һ��Ca(OH)2��Һ��ϡ��ˮ�ֱ��1L1mol��L-1��ϡ����ǡ����ȫ��Ӧ���䷴Ӧ�ȷֱ�Ϊ����1������2������3��������1������2������3�Ĵ�С��ϵΪ___��
II.ʵ��������һδ֪Ũ�ȵ�ϡ���ᣬijѧ��Ϊ�ⶨ�����Ũ����ʵ�����н�������ʵ�顣�������������գ�
����һ������250mL0.1000mol/L NaOH����Һ��
�������ȡ20.00mL����ϡ���������ƿ�У����μ�2�C3�η�̪��Һ��ָʾ�������Լ����Ƶı�NaOH��Һ���еζ����ظ������ζ�����4�Σ���¼�������¡�
ʵ���� | NaOH��Һ��Ũ��(mol/L) | �ζ����ʱ������NaOH��Һ����(mL) | ����������Һ�����(mL) |
1 | 0.10 | 20.02 | 20.00 |
2 | 0.10 | 20.00 | 20.00 |
3 | 0.10 | 19.00 | 20.00 |
4 | 0.10 | 19.98 | 20.00 |
��1������һ��Ҫ�����������ƹ��������Ϊ___g�����Ʊ���Һ��Ҫ�õ����������ձ��⣬����Ҫ�IJ���������___��
��2�������������ݣ��ɼ�����������Ũ��Ϊ___(����С�����2λ)��
��3��������ʵ���У����в���(����������ȷ)����ɲⶨ���(����ҺŨ��ֵ)ƫ�ߵ���__��
A.���Ʊ���Һ����ʱ����ˮ�����̶�
B.��ƿˮϴ��ֱ��װ����Һ
C.��ʽ�ζ���ˮϴ��δ�ô���ϡ������Һ��ϴ
D.�ζ������յ�ʱ�����Ӷ����ζ��ܶ���
E.��ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ
III.������ԭ�ζ�ԭ��ͬ�к͵ζ�ԭ�����ƣ�Ϊ�˲ⶨijδ֪Ũ�ȵ�NaHSO3��Һ��Ũ�ȣ�����0.1000mol/L������KMnO4��Һ���еζ����ش��������⣺
��1����ƽ���ӷ���ʽ��__MnO4-+__HSO3-+__H+=__
��2����KMnO4���еζ�ʱ��KMnO4��ҺӦװ��___�ζ����У�����ʽ���ʽ�����жϵζ��յ�������ǣ��������һ��KMnO4��Һʱ��___��