��Ŀ����
����Ŀ���л��������ۼ���ϩ��������������ʸߡ������ᡢ��ȫ���ܸߵ��ŵ㣬Ŀǰ���㷺�������������DZ�������������֡���ѧ��Ƭ�����ݶ����绰ͤ�������䡢��־�Ƶȡ�
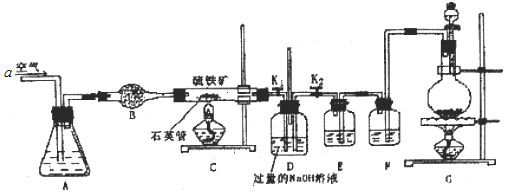
��ͼ����2������ϩΪԭ�Ϻϳ��л�����E������2������ϩ���������·�ߣ�
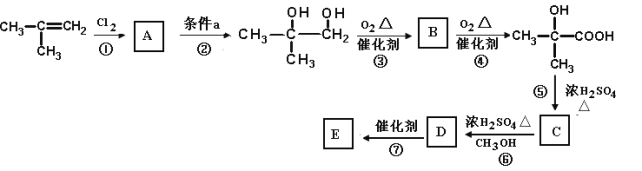
(1)�л�������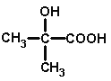 �к��еĹ�����������_____________________________________��
�к��еĹ�����������_____________________________________��
(2)д��B�Ľṹ��ʽ��___________________________________
(3)д����Ӧ�ٵĻ�ѧ����ʽ____________________________________________________________________
(4)д����Ӧ�Ļ�ѧ����ʽ__________________________________________________________________
(5)�������� ~ ��Ӧ�У�����ȡ����Ӧ����______________�����ڼӳɷ�Ӧ����___________��
(6)д��E�Ľṹ��ʽ��___________________________
(7)д���л��� ͨ�������γɸ߷��Ӿ���F�Ľṹ��ʽ��___________________________
ͨ�������γɸ߷��Ӿ���F�Ľṹ��ʽ��___________________________
���𰸡��ǻ����Ȼ� 
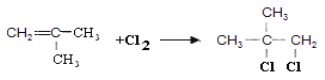 CH2=C��CH3��COOH+CH3OH
CH2=C��CH3��COOH+CH3OH![]() CH2=C��CH3��COOCH3+H2O �ڢ� ��
CH2=C��CH3��COOCH3+H2O �ڢ� �� 

��������
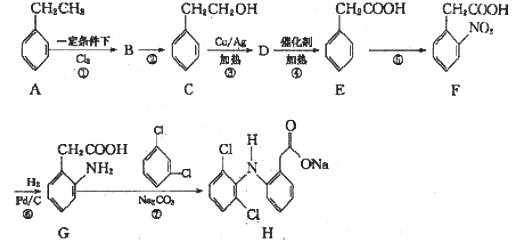
���л������ĺϳ�·��Ϊ��������������ŵ�ʶ�𡢽ṹ��ʽ���ƶϡ���ѧ����ʽ����д����Ӧ���͵��ж��ȣ����ڻ����⡣
(1)�л������� �й��������ǻ���-OH�����Ȼ���-COOH������ĿҪ��д���ơ�
�й��������ǻ���-OH�����Ȼ���-COOH������ĿҪ��д���ơ�
(2)�ݷ�Ӧ����֪����Ӧ�ۢ�Ϊ���������������л����ӷ����ı仯Ϊ-CH2OH �� -CHO �� -COOH����BΪ ��
��
(3)�ȽϷ��ӽṹ֪����Ӧ�٢�ʹ�л������й����Ŵ�һ��̼̼˫����������ǻ����ʷ�Ӧ��Ϊ�������ļӳɷ�Ӧ����ѧ����ʽΪ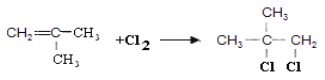 ����Ӧ��Ϊ±������ˮ�ⷴӦ������a��NaOHˮ��Һ�����ȡ�
����Ӧ��Ϊ±������ˮ�ⷴӦ������a��NaOHˮ��Һ�����ȡ�
(4)E��Ŀ������л��������߷��ӻ��������DΪ�䵥��CH2=C(CH3)COOCH3����Ӧ��Ϊ������ȥ��Ӧ��CΪCH2=C(CH3)COOH����Ӧ��Ϊ�Ȼ���״���������Ӧ����ѧ����ʽΪCH2=C��CH3��COOH+CH3OH![]() CH2=C��CH3��COOCH3+H2O��
CH2=C��CH3��COOCH3+H2O��
(5)���Ϸ�������Ӧ�� ~ ���У�����ȡ����Ӧ�����ڢ������ڼӳɷ�Ӧ������������������Ӧ�����ۢ���������ȥ��Ӧ�����ݡ�
(6)D��CH2=C(CH3)COOCH3���Ӿ۷�Ӧ����EΪ ��
��
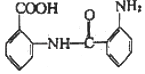
(7)�л��� ���������ǻ����Ȼ����ɷ������Ӽ������γɸ߷��Ӿ�������F�ṹ��ʽΪ��
���������ǻ����Ȼ����ɷ������Ӽ������γɸ߷��Ӿ�������F�ṹ��ʽΪ�� ��
��

 Ŀ�����ϵ�д�
Ŀ�����ϵ�д�