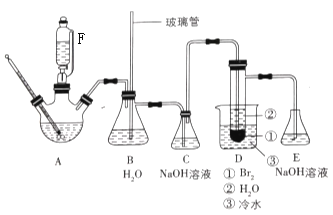
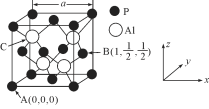
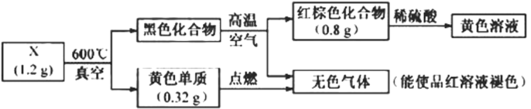
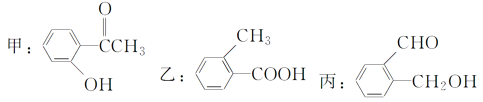
��Ŀ����
����Ŀ����1��д��SO3���ӵĿռ乹����____����____���ӣ����������������Ǽ��������������ĵȵ�����Ļ�ѧʽ��һ��������______ (д��һ��)�����ǵ�����ԭ�Ӳ��õ��ӻ���ʽ����_______��
��2����ȩ(H2C��O)��Ni�������¼���ɵü״�(CH3OH)���״�������Cԭ�ӵ��ӻ���ʽΪ__���״������ڵ�O��C��H����___����������������������������ȩ�����ڵ�O��C��H���ǣ��״���������ˮ������Ҫԭ����_____��
��3����֪�ߵ�����������ʽ����ѧʽ�ֱ�ΪH5IO6��HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᡣ��Ƚ϶�������ǿ����H5IO6_____��������������������������HIO4��
���𰸡�ƽ���������� �Ǽ��� NO3- sp2 sp3 < �״��Ǽ��Է��ӣ��и���ˮ���ţ���ˮ����γ����������������ˮ <
��������
��1��������������м۲���ӶԸ���=��������+�µ��ӶԸ���=3+![]() ��6-3��2��=3��sp2�ӻ��������µ��Ӷԣ�����Ϊƽ�������νṹ��������������غϣ�Ϊ�Ǽ��Է��ӣ�SO3��������4��ԭ�ӣ�40�����ӣ����以Ϊ�ȵ������һ��������ΪNO3-��NO3-������ԭ��Nԭ���γ�3���������¶Ե�������
��6-3��2��=3��sp2�ӻ��������µ��Ӷԣ�����Ϊƽ�������νṹ��������������غϣ�Ϊ�Ǽ��Է��ӣ�SO3��������4��ԭ�ӣ�40�����ӣ����以Ϊ�ȵ������һ��������ΪNO3-��NO3-������ԭ��Nԭ���γ�3���������¶Ե�������![]() ��0����������ԭ��Ϊsp2�ӻ����ʴ�Ϊ��ƽ���������Σ��Ǽ��ԣ�NO3-��sp2��
��0����������ԭ��Ϊsp2�ӻ����ʴ�Ϊ��ƽ���������Σ��Ǽ��ԣ�NO3-��sp2��
��2���״�������Cԭ�Ӽ۲���ӶԸ���=0+4=4������Cԭ�ӵ��ӻ���ʽΪsp3�ӻ����״�������Cԭ�ӵ��ӻ���ʽΪsp3�ӻ�������O-C-H����ԼΪ109��28������ȩ�����ڵ�Cԭ�ӵ��ӻ���ʽΪsp2�ӻ���O-C-H����ԼΪ120�������Լ״������ڵ�O-C-H����С�ڼ�ȩ�����ڵ�O-C-H���ǣ��״���������ˮ������Ҫԭ���Ǽ״��Ǽ��Է��ӣ��и���ˮ���ţ���ˮ����γ����������������ˮ���ʴ�Ϊ��sp3��<���״��Ǽ��Է��ӣ��и���ˮ���ţ���ˮ����γ����������������ˮ
��3���������з��ǻ���Խ�࣬����Խǿ��H5IO6Ϊ��Ԫ�ᣬH5IO6�к���5����OH��1�����ǻ�����HIO4ΪһԪ�ᣬ����1����OH��3�����ǻ��������H5IO6������С��HIO4���ʴ�Ϊ��<��

 �����ܿ����ϵ�д�
�����ܿ����ϵ�д�����Ŀ����֪X��Y��Z��W��Ϊ��ѧ��ѧ�г����ĵ��ʻ������֮���ת����ϵ��ͼ��ʾ(���ֲ�������ȥ)����W��X��������
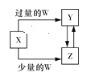
ѡ�� | A | B | C | D |
X | C | Fe | Ca(OH)2��Һ | AlCl3 |
W | O2 | HNO3 | CO2 | NH3��H2O |
A.AB.BC.CD.D