��Ŀ����
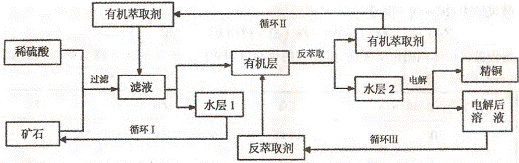
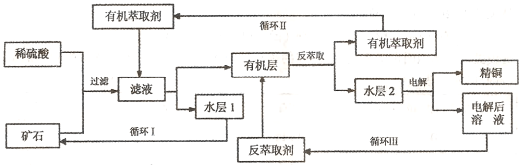
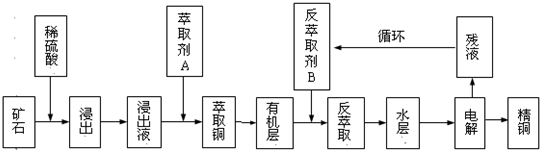
ijͭ��ʯ������ͭ��������ͭ����������������ʯ��SiO2�����ֲ���������ӿ�ʯ����ȡͭ���乤������ͼ��ͼ������ͭ����ȡ��ͭ��ˮ������л���Ĺ��̣��ͷ���ȡ��ͭ���л������ˮ��Ĺ��̣����ִ�ʪ����ͭ����Ҫ�����ֶΣ�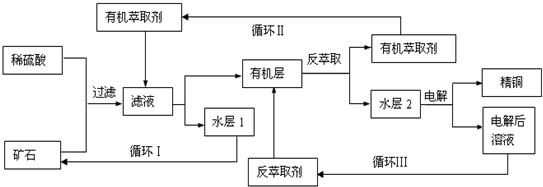
��֪����Cu2O+2H+=Cu2++Cu+H2O���ڵ���ʯ����������������̫��ʱ������������������Ļ��Һ����ͭ���۷���ȡ���ˮ��2������ͭ��Һ��
�ش��������⣺
��1����ʯ��ϡ���ᴦ�������з�����Ӧ�����ӷ���ʽΪ��Cu2O+2H+=Cu2++Cu+H2O��
���˺����õ���������NaOH��Һ��������Ӧ�Ļ�ѧ����ʽ
��2����ѭ�������ѭ�����ˮ��1���ܼ���ѭ��ʹ�ã����ɷ����һ����Ҫ�������ξ��壬�þ���Ļ�ѧʽ��
��3��д�������������������Ե缫��������Ӧ�ĵ缫��Ӧʽ��
��4����ѭ�����з���ȡ������Ҫ�ɷ���

��֪����Cu2O+2H+=Cu2++Cu+H2O���ڵ���ʯ����������������̫��ʱ������������������Ļ��Һ����ͭ���۷���ȡ���ˮ��2������ͭ��Һ��
�ش��������⣺
��1����ʯ��ϡ���ᴦ�������з�����Ӧ�����ӷ���ʽΪ��Cu2O+2H+=Cu2++Cu+H2O��
CuO+2H+�TCu2++H2O
CuO+2H+�TCu2++H2O
��Fe2O3+6H+�T2Fe3++3H2O��Cu+2Fe3+�T2Fe2++Cu2+��
Fe2O3+6H+�T2Fe3++3H2O��Cu+2Fe3+�T2Fe2++Cu2+��
����д����2���������˺����õ���������NaOH��Һ��������Ӧ�Ļ�ѧ����ʽ
SiO2+2NaOH�TNa2SiO3+H2O
SiO2+2NaOH�TNa2SiO3+H2O
���ܽ�����Һ�׳�ˮ����
ˮ����
����2����ѭ�������ѭ�����ˮ��1���ܼ���ѭ��ʹ�ã����ɷ����һ����Ҫ�������ξ��壬�þ���Ļ�ѧʽ��
FeSO4?7H2O
FeSO4?7H2O
����ˮ��1��¶�ڿ�����һ��ʱ����Եõ���һ����Ҫ�������Σ�д��ˮ��1��¶�ڿ����з�����Ӧ�����ӷ���ʽ4Fe2++O2+4H+�T4Fe3++2H2O
4Fe2++O2+4H+�T4Fe3++2H2O
����3��д�������������������Ե缫��������Ӧ�ĵ缫��Ӧʽ��
4OH--4e-�TO2+2H2O
4OH--4e-�TO2+2H2O
����4����ѭ�����з���ȡ������Ҫ�ɷ���
H2SO4
H2SO4
����������1������ͭ�����������������Ժ�ǿ�ᷢ����Ӧ�����κ�ˮ������ͭ��������֮�䷢��������ԭ��Ӧ������������������������Ժ�ǿ�Ӧ�����κ�ˮ�������Ƶ�ˮ��Һ�׳�ˮ������
��2��һ�������£���������������FeSO4?7H2O����ʽ���ڣ����������ױ�����Ϊ���������ӣ�
��3���������ͭʱ�������������������ӷ���ʧ���ӵ�������Ӧ��
��4������ѭ��ͼ�ҳ�ѭ�������ȡ����
��2��һ�������£���������������FeSO4?7H2O����ʽ���ڣ����������ױ�����Ϊ���������ӣ�
��3���������ͭʱ�������������������ӷ���ʧ���ӵ�������Ӧ��
��4������ѭ��ͼ�ҳ�ѭ�������ȡ����
����⣺��1������ͭ�����������������Ժ�ǿ�ᷢ����Ӧ�����κ�ˮ��CuO+2H+�TCu2++H2O��Fe2O3+6H+�T2Fe3++3H2O������ͭ��������֮�䷢��������ԭ��Ӧ����Cu+2Fe3+�T2Fe2++Cu2+������������������������Ժ�ǿ�Ӧ�����κ�ˮ��SiO2+2NaOH�TNa2SiO3+H2O�������Ƶ�ˮ��Һ�׳�ˮ������
�ʴ�Ϊ��CuO+2H+�TCu2++H2O��Fe2O3+6H+�T2Fe3++3H2O��Cu+2Fe3+�T2Fe2++Cu2+ ����д�������ɣ���SiO2+2NaOH�TNa2SiO3+H2O��ˮ������
��2����ѭ�������ѭ�����ˮ��1���ܼ���ѭ��ʹ�ã����ɷ����һ����Ҫ�ľ���FeSO4?7H2O����¶�ڿ�����һ��ʱ��ɱ�����������4Fe2++O2+4H+�T4Fe3++2H2O��
�ʴ�Ϊ��FeSO4?7H2O��4Fe2++O2+4H+�T4Fe3++2H2O��
��3���������ͭʱ�������������������ӷ���ʧ���ӵ�������Ӧ����4OH--4e-�TO2+2H2O���ʴ�Ϊ��4OH--4e-�TO2+2H2O��
��4����ѭ�����з���ȡ������Ҫ�ɷ������ᣬ�ʴ�Ϊ��H2SO4��
�ʴ�Ϊ��CuO+2H+�TCu2++H2O��Fe2O3+6H+�T2Fe3++3H2O��Cu+2Fe3+�T2Fe2++Cu2+ ����д�������ɣ���SiO2+2NaOH�TNa2SiO3+H2O��ˮ������
��2����ѭ�������ѭ�����ˮ��1���ܼ���ѭ��ʹ�ã����ɷ����һ����Ҫ�ľ���FeSO4?7H2O����¶�ڿ�����һ��ʱ��ɱ�����������4Fe2++O2+4H+�T4Fe3++2H2O��
�ʴ�Ϊ��FeSO4?7H2O��4Fe2++O2+4H+�T4Fe3++2H2O��
��3���������ͭʱ�������������������ӷ���ʧ���ӵ�������Ӧ����4OH--4e-�TO2+2H2O���ʴ�Ϊ��4OH--4e-�TO2+2H2O��
��4����ѭ�����з���ȡ������Ҫ�ɷ������ᣬ�ʴ�Ϊ��H2SO4��
������������һ��ʵ�鷽���������Ŀ��Ҫ��ѧ�����з����ͽ��������������ѶȽϴ�

��ϰ��ϵ�д�
 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д�
�����Ŀ
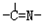 ���ֻ��ţ���״�ṹ�����ֻ��ŵ���Ŀ��ϵΪn3=
���ֻ��ţ���״�ṹ�����ֻ��ŵ���Ŀ��ϵΪn3=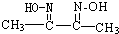
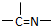 ���ֻ��ţ���״�ṹ�����ֻ��ŵ���Ŀ��ϵΪn3=
���ֻ��ţ���״�ṹ�����ֻ��ŵ���Ŀ��ϵΪn3=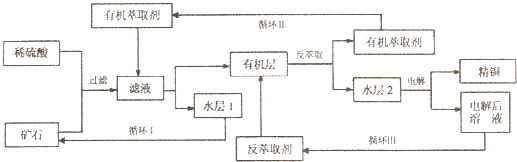
 ���ֻ��ţ���״�ṹ�����ֻ��ŵ���Ŀ��ϵΪn3=
���ֻ��ţ���״�ṹ�����ֻ��ŵ���Ŀ��ϵΪn3=