��Ŀ����
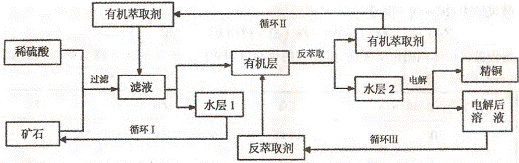
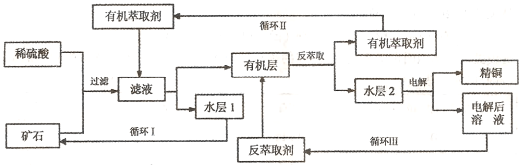
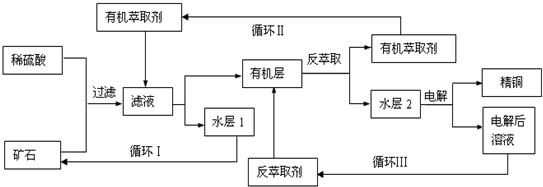
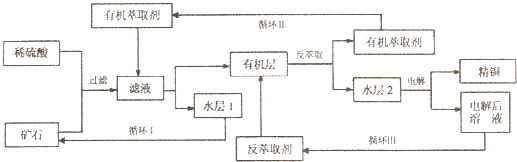
ijͭ��ʯ������ͭ��������ͭ�������������ʹ�����ʯ��SiO2�����ֲ���������ӿ�ʯ����ȡͭ��������ͼ���£�
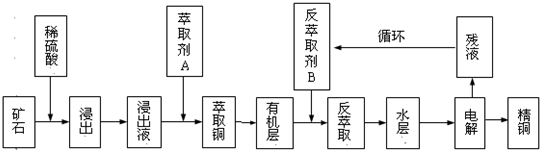
��֪���ٵ���ʯ����������������̫��ʱ������������������Ļ��Һ����ͭ���ڷ���ȡ���ˮ��������ͭ��Һ��Cu2+Ũ��ԼΪ50g/L���ش��������⣺
��1����ʯ��ϡ�������������������ͭ�����ķ�ӦΪ��Cu2O+2H+�TCu2++Cu+H2O����д���ù����з�������һ��������ԭ��Ӧ�����ӷ���ʽ��
��2����ȡ��A��һ�����Ϊ�����л��������N-510��N-530�ȣ�ij������A�ķ��ӽṹ�н���n1��-CH3��n2��-OH ��n3��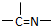 ���ֻ��ţ���״�ṹ�����ֻ��ŵ���Ŀ��ϵΪn3=
���ֻ��ţ���״�ṹ�����ֻ��ŵ���Ŀ��ϵΪn3=
��3��д�������������������Ե缫��������Ӧ�ĵ缫��Ӧʽ��
��4��ѭ���з���ȡ��B����Ҫ�ɷ���
��5��ijͭ��ʯ��Ʒ�У�������������ͭ����������������ʯ�������ʣ�ȡ�ÿ�ʯ��Ʒ200.0g����100mL1.0mol?L-1H2SO4��Һ��ȡ�������10mL 1.0mol?L-1 Fe2��SO4��3��Һ����ʹͭȫ����������ȡҺ����ֵ���ɵõ� 6.4gCu����ͭ��ʯ��Ʒ��������ͭ��������������������

��֪���ٵ���ʯ����������������̫��ʱ������������������Ļ��Һ����ͭ���ڷ���ȡ���ˮ��������ͭ��Һ��Cu2+Ũ��ԼΪ50g/L���ش��������⣺
��1����ʯ��ϡ�������������������ͭ�����ķ�ӦΪ��Cu2O+2H+�TCu2++Cu+H2O����д���ù����з�������һ��������ԭ��Ӧ�����ӷ���ʽ��
Cu+2Fe3+�T2Fe2++Cu2+
Cu+2Fe3+�T2Fe2++Cu2+
����2����ȡ��A��һ�����Ϊ�����л��������N-510��N-530�ȣ�ij������A�ķ��ӽṹ�н���n1��-CH3��n2��-OH ��n3��
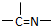 ���ֻ��ţ���״�ṹ�����ֻ��ŵ���Ŀ��ϵΪn3=
���ֻ��ţ���״�ṹ�����ֻ��ŵ���Ŀ��ϵΪn3=n1+n2-2
n1+n2-2
����A����Է�������Ϊ116�����ӽṹ��̼ԭ�Ӹ�̼ԭ�������ӣ���A�Ľṹ��ʽ��CH3-C��=N-OH��-C��=N-OH��-CH3
CH3-C��=N-OH��-C��=N-OH��-CH3
����3��д�������������������Ե缫��������Ӧ�ĵ缫��Ӧʽ��
4OH--4e-�TO2��+2H2O
4OH--4e-�TO2��+2H2O
����4��ѭ���з���ȡ��B����Ҫ�ɷ���
H2SO4
H2SO4
����5��ijͭ��ʯ��Ʒ�У�������������ͭ����������������ʯ�������ʣ�ȡ�ÿ�ʯ��Ʒ200.0g����100mL1.0mol?L-1H2SO4��Һ��ȡ�������10mL 1.0mol?L-1 Fe2��SO4��3��Һ����ʹͭȫ����������ȡҺ����ֵ���ɵõ� 6.4gCu����ͭ��ʯ��Ʒ��������ͭ��������������������
��������1������ͭ�����������������Ժ�ǿ�ᷢ����Ӧ�����κ�ˮ������ͭ��������֮�䷢��������ԭ��Ӧ��
��2�������л��������Ǿ���뿻���C=NOH�����л������
��3����⾫��ͭ�������ŵ�������OH-��
��4������ȡ���ˮ��������ͭ��Һ���ɵ��֪�������ᣬ����ѭ���з���ȡ��B�����
��5�����ݷ�Ӧ������ͭԪ���غ����������ͭ����������������ԭ��Ӧ�ĵ����غ����������������������
��2�������л��������Ǿ���뿻���C=NOH�����л������
��3����⾫��ͭ�������ŵ�������OH-��
��4������ȡ���ˮ��������ͭ��Һ���ɵ��֪�������ᣬ����ѭ���з���ȡ��B�����
��5�����ݷ�Ӧ������ͭԪ���غ����������ͭ����������������ԭ��Ӧ�ĵ����غ����������������������
����⣺��1����ʯ��ϡ�������������������ͭ�����ķ�ӦΪ��Cu2O+2H+�TCu2++Cu+H2O������ͭ��������֮�䷢��������ԭ��Ӧ����Ӧ�����ӷ���ʽΪ��Cu+2Fe3+�T2Fe2++Cu2+��
�ʴ�Ϊ����Cu+2Fe3+�T2Fe2++Cu2+��
��2��-CH3��-OH�Ƕ�ͷ����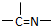 �Ƿ��ӵĹǼܣ�һ��
�Ƿ��ӵĹǼܣ�һ��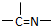 �����һ����ͷ������������β��������ͷ���������ͷ��n3+2����n3+2=n1+n2��n3=n1+n2-2��
�����һ����ͷ������������β��������ͷ���������ͷ��n3+2����n3+2=n1+n2��n3=n1+n2-2��
���ݷ����жϳ���ȡ��������A����Է�������Ϊ116�����ӽṹ��̼ԭ�Ӹ�̼ԭ�������ӣ��õ��ṹ��ʽΪ��CH3-C��=N-OH��-C��=N-OH��-CH3��
�ʴ�Ϊ��n1+n2-2��CH3-C��=N-OH��-C��=N-OH��-CH3��
��3�������������������Ե缫���ŵ��������������ʧ��������������������Ӧ�ĵ缫��Ӧʽ��4OH--4e-�TO2��+2H2O��
�ʴ�Ϊ��4OH--4e-�TO2��+2H2O��
��4������ȡ���ˮ��������ͭ��Һ���ɵ��֪�������ᣬ����ѭ���з���ȡ��B�����
�ʴ�Ϊ��H2SO4��
��5��ȡ�ÿ�ʯ��Ʒ200.0g����100mL1.0mol?L-1H2SO4��Һ��ȡ�������10mL 1.0mol?L-1 Fe2��SO4��3��Һ����ʹͭȫ����������ȡҺ����ֵ���ɵõ� 6.4gCu��
����ͭԪ���غ�������ͭ�е�ͭԪ���������ͭ��ͭCu���ʵ���=
=0.1mol��������ͭ���ʵ���Ϊ0.05mol������Ϊ0.05mol��144g/mol=7.2g��
Cu2O%=
��100%=3.6%������������ԭ��Ӧ�����غ�Fe3+��Cu+��Cu����õ���n��Fe3+��=0.1mol��ԭ��Ʒ����Ԫ�����ʵ���Ϊ=0.1mol-0.02mol=0.08mol��
����Fe2O3���ʵ���Ϊ��0.04mol����������=
��100%=3.2%��
�ʴ�Ϊ��Cu2O��3.6%�� Fe2O3��3.2%��
�ʴ�Ϊ����Cu+2Fe3+�T2Fe2++Cu2+��
��2��-CH3��-OH�Ƕ�ͷ����
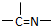 �Ƿ��ӵĹǼܣ�һ��
�Ƿ��ӵĹǼܣ�һ��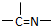 �����һ����ͷ������������β��������ͷ���������ͷ��n3+2����n3+2=n1+n2��n3=n1+n2-2��
�����һ����ͷ������������β��������ͷ���������ͷ��n3+2����n3+2=n1+n2��n3=n1+n2-2�� ���ݷ����жϳ���ȡ��������A����Է�������Ϊ116�����ӽṹ��̼ԭ�Ӹ�̼ԭ�������ӣ��õ��ṹ��ʽΪ��CH3-C��=N-OH��-C��=N-OH��-CH3��
�ʴ�Ϊ��n1+n2-2��CH3-C��=N-OH��-C��=N-OH��-CH3��
��3�������������������Ե缫���ŵ��������������ʧ��������������������Ӧ�ĵ缫��Ӧʽ��4OH--4e-�TO2��+2H2O��
�ʴ�Ϊ��4OH--4e-�TO2��+2H2O��
��4������ȡ���ˮ��������ͭ��Һ���ɵ��֪�������ᣬ����ѭ���з���ȡ��B�����
�ʴ�Ϊ��H2SO4��
��5��ȡ�ÿ�ʯ��Ʒ200.0g����100mL1.0mol?L-1H2SO4��Һ��ȡ�������10mL 1.0mol?L-1 Fe2��SO4��3��Һ����ʹͭȫ����������ȡҺ����ֵ���ɵõ� 6.4gCu��
����ͭԪ���غ�������ͭ�е�ͭԪ���������ͭ��ͭCu���ʵ���=
| 6.4g |
| 64g/mol |
Cu2O%=
| 7.2g |
| 200g |
����Fe2O3���ʵ���Ϊ��0.04mol����������=
| 0.04mol��160g/mol |
| 200g |
�ʴ�Ϊ��Cu2O��3.6%�� Fe2O3��3.2%��
���������⿴�ƿ���������ӿ�ʯ����ȡͭ����һ�����ӵĹ������̣�ʵ���Ͽ����˳����������仯��������ʡ���⾫��ͭ�������л���ṹ�Ľṹ��������Ŀ�ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
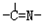 ���ֻ��ţ���״�ṹ�����ֻ��ŵ���Ŀ��ϵΪn3=
���ֻ��ţ���״�ṹ�����ֻ��ŵ���Ŀ��ϵΪn3=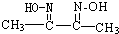
 ���ֻ��ţ���״�ṹ�����ֻ��ŵ���Ŀ��ϵΪn3=
���ֻ��ţ���״�ṹ�����ֻ��ŵ���Ŀ��ϵΪn3=