��Ŀ����
��̼���ƣ�Na2CO4����ϡ���ᷴӦ�Ļ�ѧ����ʽΪ��2Na2CO4 +2H2SO4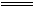 2Na2SO4+O2��+2H2O+2CO2��
2Na2SO4+O2��+2H2O+2CO2��
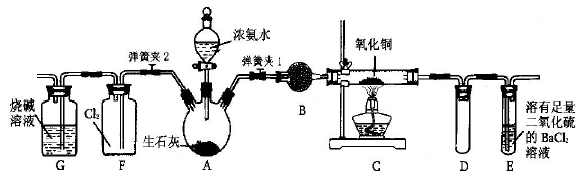
ij��̼������Ʒ�к�������Na2O2���ס�����λͬѧ��������Ϊmg�ĸ���Ʒ��ѡ����ͼ��ʾ��������ϳɲⶨ��Ʒ�Ĵ��ȵ�װ�á�����������˳���Ǣ٢ߢۢޢܣ����Ǣ٢ڡ�
��1����ͬѧ��ͨ��ʵ���õ������� ��������װ�õ�˳����ڴ������ķ����� ��
��2����ͬѧ��ͨ��ʵ���õ������� ��������õ����ݼ��������ʵ���� ���ƫ�ߡ�����ƫ�͡�����Ӱ�족���������� ��
��3��Ϊ�˲��ȷ��ʵ�����ݣ����㽫��ͬѧ��ʵ��װ�ý��иĽ���д�����ӿڵ�����˳�� ��
��4������Ľ���ʵ��װ�ý���ʵ�飬�����ʵ��ǰ��װ�âڵ������ֱ���W1g��W2g������Ʒ�й�̼���Ƶ���������Ϊ ��
 2Na2SO4+O2��+2H2O+2CO2��
2Na2SO4+O2��+2H2O+2CO2��ij��̼������Ʒ�к�������Na2O2���ס�����λͬѧ��������Ϊmg�ĸ���Ʒ��ѡ����ͼ��ʾ��������ϳɲⶨ��Ʒ�Ĵ��ȵ�װ�á�����������˳���Ǣ٢ߢۢޢܣ����Ǣ٢ڡ�


��1����ͬѧ��ͨ��ʵ���õ������� ��������װ�õ�˳����ڴ������ķ����� ��
��2����ͬѧ��ͨ��ʵ���õ������� ��������õ����ݼ��������ʵ���� ���ƫ�ߡ�����ƫ�͡�����Ӱ�족���������� ��
��3��Ϊ�˲��ȷ��ʵ�����ݣ����㽫��ͬѧ��ʵ��װ�ý��иĽ���д�����ӿڵ�����˳�� ��
��4������Ľ���ʵ��װ�ý���ʵ�飬�����ʵ��ǰ��װ�âڵ������ֱ���W1g��W2g������Ʒ�й�̼���Ƶ���������Ϊ ��
��1��CO2��������O2����� ����۽���λ��







��2��CO2������ ƫ�� ��ʯ��������ˮ����
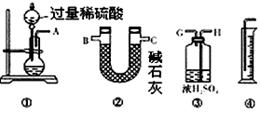
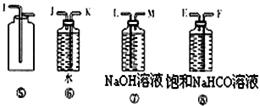
��3��A��H��G��B ��4��








��2��CO2������ ƫ�� ��ʯ��������ˮ����
��3��A��H��G��B ��4��

ʵ���Ŀ���DzⶨNa2CO4��Ʒ�Ĵ��ȣ����ܺ�������Na2O2��Na2CO4��ϡ���ᷴӦ��Na2O2��ϡ���ᷴӦ����O2�ų���ʵ���ͨ���ⶨCO2������������Na2CO4�Ĵ��ȣ����ü�ʯ������CO2֮ǰ�����ȳ�ȥCO2�е�ˮ����������ʵ���õ����ݻ�ƫ�ߣ��ʼ�ͬѧ��ʵ���Т����Ӧ����λ�ã���ͬѧӦ���Ӣ�Ũ���ᡣ

��ϰ��ϵ�д�
 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д� ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�
�����Ŀ
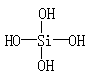 �����������õ�H6Si2O7���ӵĽṹ�У����еĹ�������ĿΪ�� ��
�����������õ�H6Si2O7���ӵĽṹ�У����еĹ�������ĿΪ�� ��